Nylon 6 Is Addition Or Condensation Polymer
News Leon
Mar 19, 2025 · 5 min read
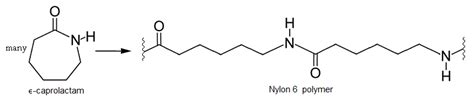
Table of Contents
Nylon 6: Addition or Condensation Polymer? Understanding Polymerization Mechanisms
The question of whether Nylon 6 is an addition or condensation polymer is a fundamental one in polymer chemistry, often causing confusion among students and even professionals. While seemingly simple, the answer requires a deep understanding of the polymerization mechanisms involved. This comprehensive article will delve into the intricacies of Nylon 6 synthesis, clarifying its classification and exploring the key differences between addition and condensation polymerization.
Understanding Polymerization: Addition vs. Condensation
Before diving into the specifics of Nylon 6, let's establish a clear understanding of the two main types of polymerization:
Addition Polymerization
Addition polymerization involves the sequential addition of monomers to a growing polymer chain without the loss of any atoms. This process typically occurs via a chain reaction mechanism involving initiation, propagation, and termination steps. The monomers usually contain carbon-carbon double bonds (alkenes) that undergo opening to form new single bonds within the polymer chain. Examples of polymers formed via addition polymerization include polyethylene (PE), polypropylene (PP), and polyvinyl chloride (PVC). Key characteristics include:
- No small molecule byproduct: No atoms are lost during the polymerization process.
- Monomer with unsaturated bonds: Usually involves monomers with carbon-carbon double or triple bonds.
- Chain reaction mechanism: Initiation, propagation, and termination steps are involved.
Condensation Polymerization
Condensation polymerization, on the other hand, involves the joining of monomers with the simultaneous elimination of a small molecule, such as water, methanol, or HCl. This process often requires functional groups on the monomers capable of reacting with each other, such as carboxylic acids and amines. The resulting polymer chain has a different chemical composition than the sum of its monomers due to the loss of the small molecule byproduct. Examples include polyesters (like PET), polyamides (like Nylon 6,6), and polycarbonates. Key characteristics include:
- Small molecule byproduct: A small molecule is released during the polymerization process (e.g., water).
- Monomers with reactive functional groups: Monomers possess functional groups that react to form the polymer chain.
- Step-growth mechanism: The polymerization proceeds in a step-wise manner.
The Synthesis of Nylon 6: A Closer Look
Nylon 6 is a polyamide, a type of polymer characterized by amide linkages (-CONH-) connecting the monomer units. Unlike Nylon 6,6 (which is a condensation polymer), Nylon 6's synthesis is more nuanced. It's primarily produced via ring-opening polymerization of caprolactam, a cyclic amide. This process can be considered a variation of both addition and condensation polymerization.
The reaction proceeds through several steps:
-
Ring Opening: Caprolactam, a six-membered cyclic amide, undergoes ring opening initiated by water or a small amount of acid. This step creates a reactive amino acid, aminocaproic acid.
-
Chain Growth: The aminocaproic acid reacts with another caprolactam molecule, opening its ring and forming an amide bond. This step continues to extend the chain. This growth step is characterized by the formation of an amide bond, which is a characteristic of condensation polymerization.
-
Chain Propagation: The process continues, with each newly opened caprolactam molecule adding to the growing polymer chain.
-
Chain Termination: The reaction is typically terminated by reaching equilibrium or by exhaustion of the starting material.
Why Nylon 6 is Considered a Condensation Polymer (with nuances)
While Nylon 6's synthesis involves ring opening, a characteristic of addition polymerization, the formation of the amide linkage involves the elimination of a water molecule. Each amide bond formed releases a molecule of water. This water molecule is typically removed from the reaction mixture during the polymerization process.
Therefore, Nylon 6 is classified as a condensation polymer, even though its synthesis utilizes ring-opening polymerization. This classification is based on the net result of the polymerization reaction: the formation of an amide bond accompanied by the elimination of a small molecule.
Contrasting Nylon 6 Synthesis with typical addition polymerization
The key difference lies in the nature of the bond formation and the presence or absence of a byproduct. In a typical addition polymerization, monomers add to the chain without any loss of atoms. Nylon 6's synthesis, while involving ring-opening, forms the amide bonds by the reaction of the amino and carboxyl groups, leading to water removal – a defining feature of condensation polymerization.
The Importance of Understanding Polymer Classification
Accurately classifying polymers as addition or condensation is crucial for several reasons:
-
Predicting Polymer Properties: The polymerization mechanism influences the final polymer's properties. Condensation polymers often have higher melting points and stronger intermolecular forces due to hydrogen bonding or dipole-dipole interactions, often resulting from the presence of polar functional groups involved in the condensation reaction.
-
Understanding Reaction Kinetics: The rate and mechanism of polymerization affect the molecular weight and distribution of the polymer, impacting its physical and mechanical properties.
-
Optimizing Polymer Synthesis: Understanding the mechanisms involved allows for precise control over reaction conditions and the resulting polymer characteristics.
-
Material Selection: This knowledge is essential in choosing the right polymer for specific applications based on required properties, like flexibility, strength, thermal stability, and chemical resistance.
Applications of Nylon 6
Nylon 6's unique properties, stemming from its polyamide structure and synthesis method, have made it a versatile material with a wide range of applications, including:
-
Textiles: Nylon 6 is used extensively in the production of fibers for clothing, carpets, and other textiles. Its strength, elasticity, and wrinkle resistance make it a popular choice.
-
Packaging: Its strength and barrier properties make it suitable for films and packaging applications.
-
Automotive: Used in various components like gears, bearings, and fuel lines, due to its high strength and resistance to wear and tear.
-
Electrical Engineering: Its insulating properties and resistance to chemicals make it suitable for electrical connectors and components.
-
Medical Applications: Biocompatibility of certain formulations makes it appropriate for surgical sutures and some implants.
Conclusion: A nuanced perspective
In conclusion, while Nylon 6's synthesis involves ring-opening polymerization, the net result of the reaction – the formation of amide bonds with the concomitant elimination of water – firmly places it in the category of condensation polymers. This nuanced understanding is vital for comprehending its properties, synthesis, and vast applications in diverse industries. The seeming contradiction highlights the importance of analyzing the fundamental chemical transformations that occur during polymerization, not merely focusing on the apparent mechanisms. This detailed exploration provides a clear and comprehensive understanding of Nylon 6's classification and the broader context of polymer chemistry.
Latest Posts
Latest Posts
-
What Set Of Reflections Would Carry Hexagon Abcdef Onto Itself
Mar 19, 2025
-
Is Aluminum Hydroxide Soluble In Water
Mar 19, 2025
-
Which Element Has Chemical Properties Most Similar To Sodium
Mar 19, 2025
-
Is Supporting Combustion A Physical Or Chemical Property
Mar 19, 2025
-
What Class Lever Is A Wheelbarrow
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Nylon 6 Is Addition Or Condensation Polymer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
