Is Aluminum Hydroxide Soluble In Water
News Leon
Mar 19, 2025 · 5 min read
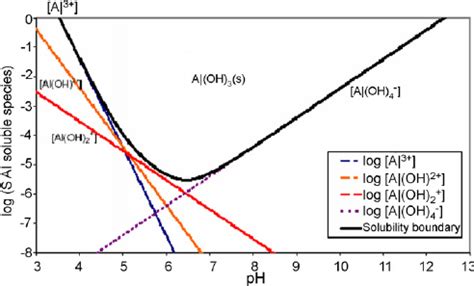
Table of Contents
Is Aluminum Hydroxide Soluble in Water? A Deep Dive into Solubility, Properties, and Applications
Aluminum hydroxide, a white, odorless, amphoteric compound with the chemical formula Al(OH)₃, is a substance that sparks considerable interest in various fields, from medicine to industrial applications. A crucial question that frequently arises is: Is aluminum hydroxide soluble in water? The answer, as with many things in chemistry, isn't a simple yes or no. This comprehensive article will explore the solubility of aluminum hydroxide, delve into its properties, examine its applications, and discuss the factors influencing its behavior in aqueous solutions.
Understanding Solubility: A Fundamental Concept
Before delving into the specifics of aluminum hydroxide, let's establish a clear understanding of solubility. Solubility refers to the maximum amount of a solute (in this case, aluminum hydroxide) that can dissolve in a given amount of solvent (water) at a specific temperature and pressure to form a saturated solution. Solubility is often expressed in grams of solute per liter of solvent (g/L) or as molarity (moles of solute per liter of solvent, mol/L).
The solubility of a compound depends on several factors including:
- The nature of the solute and solvent: Polar solutes tend to dissolve in polar solvents, while non-polar solutes dissolve in non-polar solvents. This is governed by the principle of "like dissolves like."
- Temperature: Solubility often increases with increasing temperature, although there are exceptions.
- Pressure: Pressure primarily affects the solubility of gases in liquids. It has a less significant effect on the solubility of solids in liquids.
- pH of the solution: The pH significantly impacts the solubility of amphoteric compounds like aluminum hydroxide.
The Amphoteric Nature of Aluminum Hydroxide
Aluminum hydroxide's unique amphoteric nature plays a critical role in determining its solubility. Amphoteric substances can act as both acids and bases, depending on the conditions. In acidic solutions, aluminum hydroxide behaves as a base, reacting with the H⁺ ions to form aluminum ions (Al³⁺) and water:
Al(OH)₃(s) + 3H⁺(aq) → Al³⁺(aq) + 3H₂O(l)
Conversely, in alkaline solutions, aluminum hydroxide acts as an acid, reacting with hydroxide ions (OH⁻) to form aluminate ions ([Al(OH)₄]⁻):
Al(OH)₃(s) + OH⁻(aq) → [Al(OH)₄]⁻(aq)
This amphoteric behavior significantly influences its solubility. Aluminum hydroxide exhibits low solubility in pure water, but its solubility increases substantially in both acidic and alkaline solutions due to the reactions described above.
Solubility in Pure Water: A Case of Low Solubility
In pure water, the solubility of aluminum hydroxide is extremely low, typically reported to be less than 0.0001 g/L at room temperature. This is because the dissolution process involves overcoming the strong electrostatic forces holding the Al³⁺ and OH⁻ ions together in the solid lattice. The slight solubility results from an equilibrium between the undissolved solid and the small amount of dissolved Al³⁺ and OH⁻ ions.
Factors Affecting Aluminum Hydroxide Solubility
Several factors influence the solubility of aluminum hydroxide beyond its inherent amphoteric nature:
- Temperature: Like many solids, the solubility of aluminum hydroxide increases slightly with temperature, although the increase is relatively small.
- Particle size: Finely divided aluminum hydroxide particles tend to have a higher solubility than larger particles due to the increased surface area exposed to the solvent.
- Presence of other ions: The presence of other ions in the solution can influence the solubility of aluminum hydroxide through common ion effect or complex formation. For instance, the presence of other cations or anions can compete for hydration sites, affecting the solubility.
- pH: The pH is the most significant factor influencing the solubility of aluminum hydroxide. As mentioned previously, its solubility is enhanced significantly at both low (acidic) and high (alkaline) pH values. The solubility is at a minimum near neutral pH.
Applications of Aluminum Hydroxide: Leveraging its Properties
The unique properties of aluminum hydroxide, particularly its amphoteric nature and low solubility in water, make it a valuable substance with a wide range of applications:
1. Antacids and Pharmaceuticals: Neutralizing Stomach Acid
Aluminum hydroxide's ability to neutralize stomach acid is a cornerstone of its use in antacids. When ingested, it reacts with hydrochloric acid (HCl) in the stomach, forming aluminum chloride and water, thus providing relief from heartburn and indigestion:
Al(OH)₃(s) + 3HCl(aq) → AlCl₃(aq) + 3H₂O(l)
2. Water Treatment: Removing Impurities
Aluminum hydroxide plays a crucial role in water purification. It acts as a flocculant, aiding in the removal of suspended particles, bacteria, and other impurities from water. In this application, aluminum hydroxide precipitates, forming a gelatinous hydroxide that traps contaminants, allowing for their subsequent removal by filtration or sedimentation.
3. Cosmetics and Personal Care Products: Enhancing Texture and Absorption
Aluminum hydroxide finds application in cosmetics and personal care products as an absorbent, thickener, and opacifier. Its ability to absorb moisture and oil makes it a valuable ingredient in many lotions, creams, and powders.
4. Flame Retardants: Suppressing Combustion
Aluminum hydroxide is an effective flame retardant due to its ability to release water vapor when heated. This endothermic reaction absorbs heat, thus reducing the temperature of the surrounding materials and inhibiting combustion. This property makes it useful in various materials, including plastics, textiles, and coatings.
5. Industrial Catalyst Support: Providing a Stable Base
Aluminum hydroxide acts as a support material for catalysts in various industrial processes. Its high surface area and porous structure provide a stable base for active catalytic components, enhancing their efficiency and stability.
Conclusion: A Complex Solubility Story
The question, "Is aluminum hydroxide soluble in water?" doesn't have a straightforward answer. While its solubility in pure water is extremely low, its amphoteric nature and the influence of pH drastically alter its behavior. Understanding this complex interplay of factors is crucial for effectively utilizing aluminum hydroxide in its diverse applications. Its low solubility in neutral water, coupled with its high reactivity in acidic and alkaline environments, makes it a unique and valuable compound with a multitude of uses spanning various industries and scientific fields. Further research continues to unveil new facets of aluminum hydroxide's behaviour and potential applications, further cementing its role as a crucial substance in modern chemistry and technology. The intricate dance between its solubility and its amphoteric nature continues to fascinate and challenge researchers, continually expanding our understanding of this important compound.
Latest Posts
Latest Posts
-
Which Statement Is Incorrect For The Following Reaction Profile
Mar 19, 2025
-
Difference Between Electric Potential And Electric Potential Energy
Mar 19, 2025
-
What Is 200 In Decimal Form
Mar 19, 2025
-
What Ocean Is West Of Africa
Mar 19, 2025
-
True Or False All Rational Numbers Are Integers
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Aluminum Hydroxide Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
