Which Statement Is Incorrect For The Following Reaction Profile
News Leon
Mar 19, 2025 · 6 min read
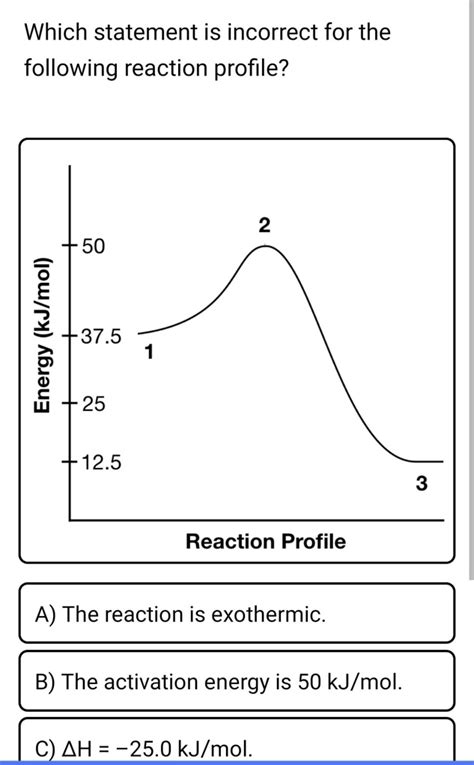
Table of Contents
Deconstructing Reaction Profiles: Identifying the Incorrect Statement
Understanding reaction profiles is crucial for grasping the kinetics and thermodynamics of chemical reactions. Reaction profiles graphically represent the energy changes that occur during a reaction, providing insights into activation energies, reaction intermediates, and overall energy changes (ΔE or ΔH). This article delves into the intricacies of analyzing reaction profiles, focusing on identifying incorrect statements about a given profile. We will explore various scenarios, highlight common misconceptions, and offer a systematic approach to accurately interpret these diagrams.
Understanding the Components of a Reaction Profile
Before we dissect incorrect statements, let's establish a firm foundation on the key elements of a typical reaction profile:
- Reactants: These are the starting materials of the reaction, positioned on the left-hand side of the diagram at a specific energy level.
- Products: These are the substances formed at the end of the reaction, located on the right-hand side at a different energy level.
- Transition State (Activated Complex): This is a high-energy, unstable intermediate species formed during the reaction. It represents the highest point on the reaction profile and corresponds to the activation energy. It is crucial to remember that the transition state is not a stable intermediate; it's a fleeting structure at the peak of the energy barrier.
- Intermediates: These are relatively stable species formed during the reaction mechanism. They appear as energy "valleys" between successive transition states in multi-step reactions. They are distinct from the reactants and products.
- Activation Energy (Ea): This is the minimum energy required for the reactants to overcome the energy barrier and proceed to form products. It's the difference in energy between the reactants and the transition state.
- ΔE (or ΔH): This represents the overall energy change of the reaction. It's the difference in energy between the reactants and the products. A negative ΔE indicates an exothermic reaction (heat is released), while a positive ΔE indicates an endothermic reaction (heat is absorbed).
Common Incorrect Statements and their Corrections
Let's examine several common incorrect statements about reaction profiles and explain why they are flawed. To illustrate, we will consider a hypothetical reaction profile for a two-step reaction:
(Imagine a diagram here showing a reaction profile with two peaks (transition states) and a valley (intermediate) between them. The reactants are at a higher energy level than the products, indicating an exothermic reaction.)
Incorrect Statement 1: "The intermediate is the most stable species in the reaction."
Correction: This statement is incorrect because the stability of a species is judged relative to other species in the reaction pathway. While the intermediate might be more stable than the transition states, it's not necessarily the most stable species. The products, in an exothermic reaction, are often the most stable species due to the lower energy level. In an endothermic reaction, the reactants are generally the more stable species.
Incorrect Statement 2: "The height of the first peak determines the overall rate of the reaction."
Correction: In a multi-step reaction, the overall rate is determined by the slowest step, which corresponds to the highest activation energy barrier. This is the rate-determining step. Even if the first step has a lower activation energy, a subsequent slower step will dictate the overall reaction rate.
Incorrect Statement 3: "The transition state is a stable intermediate."
Correction: As mentioned earlier, this is a fundamental misconception. The transition state is a high-energy, short-lived species. It's not a stable intermediate that can be isolated or observed directly. It represents the point of maximum energy along the reaction coordinate, and its lifetime is incredibly short.
Incorrect Statement 4: "The reaction profile shows the exact path taken by the reaction."
Correction: Reaction profiles are simplified representations of the reaction mechanism. They show the overall energy changes, but they don't illustrate the detailed atomic movements and rearrangements that occur during the reaction. The profile represents the most energetically favorable pathway, but other pathways might exist, though they would have higher activation energies and are therefore less likely.
Incorrect Statement 5: "The activation energy is always the same for the forward and reverse reactions."
Correction: This statement is false. The activation energy for the forward reaction (Ea,forward) is different from the activation energy for the reverse reaction (Ea,reverse). The difference between these two activation energies is related to the overall enthalpy change (ΔH) of the reaction. The relationship is: ΔH = Ea,forward – Ea,reverse.
Incorrect Statement 6: "The reaction profile is independent of temperature."
Correction: The reaction profile is influenced by temperature. While the overall energy change (ΔH) remains relatively constant with temperature changes (over a limited range), the activation energy and reaction rate are significantly affected by temperature. Higher temperatures generally lead to a greater fraction of molecules possessing sufficient energy to overcome the activation energy barrier, thus accelerating the reaction rate.
Incorrect Statement 7: "A reaction with a high activation energy will always be slow."
Correction: While a high activation energy generally indicates a slower reaction rate, other factors influence reaction speed. The concentration of reactants, presence of catalysts, and temperature all play significant roles. A reaction with a high activation energy can still proceed at a reasonable rate if other conditions are favorable (e.g., high temperatures, high concentrations).
Incorrect Statement 8: "A large negative ΔE guarantees a fast reaction."
Correction: The overall energy change (ΔE or ΔH) only tells us about the thermodynamics of the reaction, not its kinetics. A highly exothermic reaction (large negative ΔE) might still be slow if it has a high activation energy. Kinetics is concerned with the rate of the reaction, while thermodynamics focuses on the feasibility and equilibrium of the reaction.
Incorrect Statement 9: "The reaction profile always shows a single peak for a single-step reaction."
Correction: While this is often the case, there are exceptions. Some single-step reactions might exhibit a more complex profile depending on the nature of the transition state, with multiple conformations leading to multiple peaks in the reaction profile, although these might be very close together.
Incorrect Statement 10: "All reactions have intermediates."
Correction: Many reactions, especially those that occur in a single step, do not involve intermediates. Only multi-step reactions typically have intermediates appearing as valleys in the reaction profile.
Analyzing Reaction Profiles: A Step-by-Step Approach
To accurately interpret and avoid making incorrect statements about reaction profiles, follow these steps:
- Identify Reactants and Products: Determine the starting materials and the final products.
- Locate Transition States: Pinpoint the highest energy points on the profile, representing the transition states.
- Identify Intermediates (if any): Look for energy valleys between successive transition states.
- Determine Activation Energies: Calculate the energy difference between reactants/intermediates and transition states.
- Calculate the Overall Energy Change (ΔE or ΔH): Find the energy difference between the reactants and products.
- Analyze the Reaction Order: The reaction profile itself doesn't directly give reaction order, but the number of peaks (transition states) can provide clues about the number of elementary steps in a complex reaction.
- Consider the Effect of Temperature and Catalysts: Remember that temperature and catalysts can significantly influence the reaction rate but do not typically alter the overall enthalpy change (ΔH).
By carefully examining each aspect of the reaction profile and applying a systematic approach, you can effectively analyze the energy changes during a reaction and avoid making common incorrect interpretations. Remember to consider the thermodynamic and kinetic implications of the profile and avoid conflating the two. Understanding the nuances of reaction profiles is a critical skill for any chemist or chemical engineer.
Latest Posts
Latest Posts
-
Sigma And Pi Bonds In Co2
Mar 19, 2025
-
A Floating Ice Block Is Pushed Through A Displacement
Mar 19, 2025
-
What Event Had An Enormous Effect On Us Workplace Safety
Mar 19, 2025
-
What Is The Formula For Magnesium Acetate
Mar 19, 2025
-
Converse Of Alternate Exterior Angles Theorem
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Statement Is Incorrect For The Following Reaction Profile . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
