Which Element Has Chemical Properties Most Similar To Sodium
News Leon
Mar 19, 2025 · 5 min read
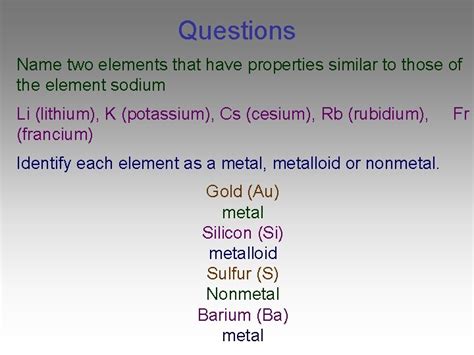
Table of Contents
Which Element Has Chemical Properties Most Similar to Sodium?
Sodium (Na), a highly reactive alkali metal, holds a unique position in the periodic table. Understanding its chemical behavior requires examining its electron configuration and its position within Group 1. This article delves into the intricacies of sodium's chemical properties and identifies the element that shares the most similarities: lithium (Li). While other alkali metals exhibit similarities, lithium's properties align most closely with sodium's due to a combination of factors, including atomic size, ionization energy, and reactivity.
Understanding Sodium's Chemical Behavior
Sodium's chemical behavior is primarily governed by its single valence electron. This lone electron in its outermost shell is easily lost, resulting in the formation of a stable +1 cation (Na⁺). This tendency to lose an electron drives sodium's high reactivity, making it readily participate in various chemical reactions.
Key Chemical Properties of Sodium:
- High Reactivity: Sodium reacts vigorously with water, producing hydrogen gas and sodium hydroxide, a highly alkaline solution. This reaction is exothermic, releasing considerable heat.
- Electropositivity: Sodium readily loses its valence electron, exhibiting a high degree of electropositivity. This means it tends to form ionic compounds with nonmetals, where it donates its electron to achieve a stable electron configuration.
- Low Ionization Energy: The ionization energy of sodium is relatively low, meaning it requires minimal energy to remove its valence electron. This contributes to its high reactivity.
- Formation of Ionic Compounds: Sodium forms ionic compounds with a wide range of nonmetals, including halogens (chlorine, bromine, iodine), oxygen, and sulfur. These compounds are typically crystalline solids with high melting points.
- Reducing Agent: Due to its tendency to lose an electron, sodium acts as a powerful reducing agent in various chemical reactions. It readily donates electrons to other substances, causing their reduction.
- Flame Color: Sodium imparts a characteristic bright yellow-orange color to a flame, a property used in analytical chemistry for its identification.
Comparing Sodium to Other Alkali Metals
The alkali metals (Group 1) all share similar chemical properties, given their single valence electron. However, the degree of similarity varies across the group due to variations in atomic size and resulting differences in ionization energy and electronegativity. Let's briefly compare sodium to other alkali metals:
- Lithium (Li): Shares the most similar properties with sodium. Both readily lose one electron to form +1 ions. However, lithium's smaller size results in slightly different reactivity and a stronger polarizing effect on anions.
- Potassium (K): Larger than sodium, potassium exhibits higher reactivity due to its larger atomic size and lower ionization energy. Its reactions are generally faster and more vigorous than those of sodium.
- Rubidium (Rb) and Cesium (Cs): Even larger than potassium, rubidium and cesium exhibit even greater reactivity and lower ionization energies. Their reactions are extremely vigorous and sometimes explosive.
Why Lithium is the Closest Match
While all alkali metals share the common trait of a single valence electron, several factors distinguish lithium and sodium:
1. Atomic Size and Ionization Energy:
Although lithium has a significantly smaller atomic radius than sodium, the difference isn't as drastic when compared to potassium, rubidium, or cesium. This moderate difference in size influences ionization energy; lithium's ionization energy is higher than sodium's but significantly lower than subsequent alkali metals. This difference is less pronounced than the differences between sodium and the heavier alkali metals.
2. Reactivity with Water:
Both lithium and sodium react vigorously with water, but the reaction of lithium is less violent than that of sodium. The less vigorous reaction is due to the formation of a protective layer of lithium hydroxide on the surface of the metal, which slows down the reaction rate compared to sodium. This is a subtle yet important difference demonstrating a degree of shared, yet distinct, reactivity.
3. Formation of Similar Compounds:
Both lithium and sodium readily form ionic compounds with similar stoichiometries and crystal structures. For instance, both form halides (LiCl, NaCl), oxides (Li₂O, Na₂O), and hydroxides (LiOH, NaOH). The similarity in the structure and properties of these compounds underscores their chemical kinship.
4. Diagonal Relationship:
Lithium exhibits a diagonal relationship with magnesium (Mg), an alkaline earth metal. This is attributed to similar ionic radii and charge densities. Although not directly explaining the similarity with sodium, it highlights the nuanced variations in properties within the periodic table, suggesting that a closer examination of this relationship reveals subtle similarities between lithium and sodium's behavior.
Other Similarities and Differences
While the similarities are substantial, some differences exist:
- Solubility: Some lithium salts exhibit different solubility properties compared to the corresponding sodium salts. This difference is attributed to the higher charge density of the smaller lithium ion.
- Polarizing Power: Lithium, being smaller, has a stronger polarizing power than sodium, influencing the properties of its compounds. This leads to deviations in some of the properties of its compounds compared to sodium's.
Conclusion
In summary, although all alkali metals share fundamental similarities due to their electron configuration, lithium (Li) possesses the closest chemical properties to sodium (Na). While differences in atomic size and resulting variations in ionization energy and reactivity exist, the overall similarity in their reactivity, compound formation, and the moderate difference in their atomic size and ionization energies compared to other alkali metals make lithium the strongest candidate. The relatively less vigorous reaction of lithium with water and its stronger polarizing power are nuances that differentiate it from sodium, but these differences are less significant when compared to the differences between sodium and the heavier alkali metals. The similarities outweigh the differences, solidifying lithium's position as the element with the most similar chemical properties to sodium.
Latest Posts
Latest Posts
-
Which Of The Following Cannot Travel In A Vacuum
Mar 19, 2025
-
What Is The Molecular Mass Of Helium
Mar 19, 2025
-
How To Add Numbers In Python
Mar 19, 2025
-
How Many Inches Are In 4 5 Feet
Mar 19, 2025
-
Does The I Band Shorten During Contraction
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has Chemical Properties Most Similar To Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
