What Is The Molecular Mass Of Helium
News Leon
Mar 19, 2025 · 5 min read
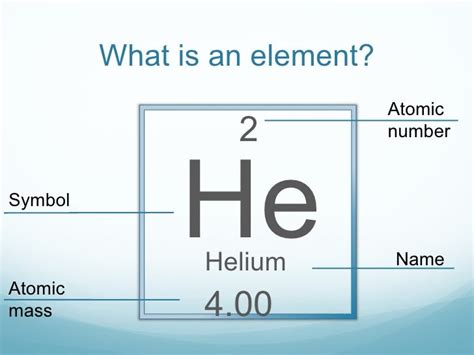
Table of Contents
What is the Molecular Mass of Helium? A Deep Dive into Atomic and Molecular Weight
Helium, the second element on the periodic table, is a fascinating noble gas known for its lightness and unique properties. Understanding its molecular mass is crucial in various scientific fields, from chemistry and physics to engineering and medicine. This article will delve deep into the concept of helium's molecular mass, explaining its calculation, applications, and the subtle nuances related to atomic versus molecular weight in the context of this unique element.
Understanding Atomic Mass and Molecular Mass
Before we dive into the specifics of helium, let's clarify the difference between atomic mass and molecular mass.
-
Atomic Mass: This refers to the mass of a single atom of an element. It's usually expressed in atomic mass units (amu), where 1 amu is approximately 1/12 the mass of a carbon-12 atom. The atomic mass is an average, taking into account the abundance of different isotopes of that element in nature. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons.
-
Molecular Mass (or Molecular Weight): This term applies to molecules, which are formed when two or more atoms chemically bond. Molecular mass is the sum of the atomic masses of all atoms in the molecule. For example, the molecular mass of water (H₂O) is the sum of the atomic mass of two hydrogen atoms and one oxygen atom.
The key difference: Atomic mass is for individual atoms; molecular mass is for molecules composed of multiple atoms. Since helium is a monatomic gas—meaning it exists as single atoms, not molecules—its atomic mass and molecular mass are effectively the same.
Calculating the Molecular Mass of Helium
Helium primarily exists as the isotope Helium-4 (⁴He), which has two protons and two neutrons. A trace amount of Helium-3 (³He) also exists, with two protons and one neutron. To calculate the average atomic (and molecular) mass of helium, we consider the relative abundance of each isotope:
-
Helium-4 (⁴He): This isotope makes up approximately 99.999863% of naturally occurring helium. Its atomic mass is approximately 4.0026 amu.
-
Helium-3 (³He): This isotope constitutes a tiny fraction (about 0.000137%) of natural helium. Its atomic mass is approximately 3.0160 amu.
The average atomic mass of helium is calculated by weighting the atomic mass of each isotope by its relative abundance:
(4.0026 amu * 0.99999863) + (3.0160 amu * 0.00000137) ≈ 4.0026 amu
Therefore, the molecular mass of helium is approximately 4.0026 amu. Since helium exists as individual atoms, the molecular mass is practically identical to its atomic mass. This slight difference is due to the minute contribution of Helium-3. For most practical purposes, 4.00 amu is a sufficiently accurate approximation.
Significance of Helium's Molecular Mass
The low molecular mass of helium has several profound implications:
1. Low Density and Buoyancy:
Helium's low molecular mass contributes to its incredibly low density. This is why helium balloons float—helium is much less dense than air, resulting in a buoyant force that overcomes the weight of the balloon. This property has numerous applications, from party balloons to lifting heavy equipment in airships (though, historically, hydrogen was also used).
2. Thermal Properties:
Helium's low mass affects its thermal properties. It has a high thermal conductivity, meaning it readily transmits heat. This makes it useful in applications requiring efficient heat transfer, such as cryogenics (cooling systems).
3. Diffusion and Permeability:
Because of its small atomic size and low mass, helium diffuses and permeates through materials much more readily than many other gases. This property is utilized in leak detection and various scientific instruments.
4. Applications in Scientific Research:
Helium's unique properties, largely stemming from its low molecular mass, are crucial in various scientific instruments like mass spectrometers, gas chromatographs, and nuclear magnetic resonance (NMR) machines. In these applications, its inertness and low mass are critical for accurate measurements and analysis.
5. Medical Applications:
Helium's low solubility in blood and its inertness make it a valuable gas in various medical applications. It’s used in some breathing mixtures for deep-sea divers and in MRI scanners to improve image quality.
6. Industrial Applications:
The low molecular mass also contributes to its use in welding, where it acts as a shielding gas, protecting the weld from atmospheric contamination. It's also used in leak detection in various industrial settings.
Distinguishing Atomic Mass from Molecular Mass in Different Contexts
While for helium, the terms are practically interchangeable, the distinction between atomic and molecular mass becomes crucial when dealing with polyatomic gases like oxygen (O₂) or carbon dioxide (CO₂).
Oxygen (O₂): The atomic mass of oxygen is approximately 16 amu. However, oxygen exists as a diatomic molecule (O₂). Therefore, its molecular mass is 2 * 16 amu = 32 amu. This significantly impacts its density and other properties.
Carbon Dioxide (CO₂): The molecular mass of carbon dioxide is calculated by summing the atomic masses of one carbon atom (approximately 12 amu) and two oxygen atoms (2 * 16 amu = 32 amu). The total molecular mass is 12 amu + 32 amu = 44 amu.
Isotopic Variations and Their Impact on Molecular Mass
The presence of different isotopes affects the average atomic mass and hence the molecular mass (when applicable). While Helium-3's contribution to the average molecular mass of helium is negligible, in other elements, isotopic variations can have a more noticeable impact. This is particularly relevant in fields such as nuclear physics and geochemistry where precise mass determination is critical.
The Importance of Accurate Molecular Mass Determination
Accurate determination of molecular mass is critical in various scientific and technological applications. Techniques such as mass spectrometry provide highly precise measurements, allowing researchers to characterize molecules and understand their properties. This accuracy is important for fields ranging from materials science to pharmaceutical development.
Conclusion:
The molecular mass of helium, approximately 4.0026 amu, is a fundamental property that underpins its unique characteristics and diverse applications. While its monatomic nature makes its atomic and molecular mass virtually identical, understanding this distinction remains crucial when working with polyatomic molecules. The low molecular mass of helium is responsible for its low density, high thermal conductivity, and ability to diffuse readily, making it an invaluable element in various scientific, technological, and medical fields. The precise measurement of molecular mass, using advanced techniques, continues to be critical for advancement in countless areas of scientific inquiry.
Latest Posts
Latest Posts
-
What Is The Distance Between The Sun And Saturn
Mar 19, 2025
-
Why Are Producers Important To The Ecosystem
Mar 19, 2025
-
Example Of A Small Scale Map
Mar 19, 2025
-
The First Fully 64 Bit Compatible Version Of Android Is
Mar 19, 2025
-
If Qc Is Greater Than Kc
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Mass Of Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
