Does The I Band Shorten During Contraction
News Leon
Mar 19, 2025 · 6 min read
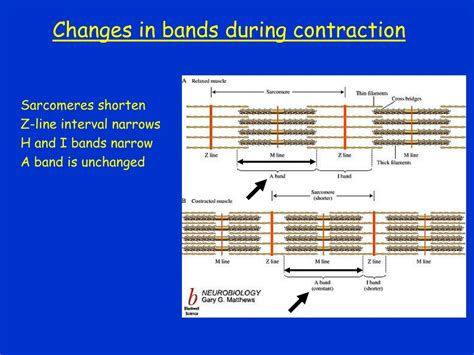
Table of Contents
Does the I Band Shorten During Contraction? Understanding Skeletal Muscle Contraction
The question of whether the I band shortens during muscle contraction is fundamental to understanding how muscles generate force. The answer, while seemingly simple, requires a deeper dive into the intricate structure and function of skeletal muscle. This article will explore the intricacies of muscle contraction, focusing specifically on the behavior of the I band during this process. We'll examine the sliding filament theory, the roles of actin and myosin, and the changes occurring within the sarcomere, the basic contractile unit of muscle.
Understanding the Sarcomere: The Basic Unit of Muscle Contraction
Before delving into the I band's behavior, let's establish a solid understanding of the sarcomere. The sarcomere is the fundamental unit of muscle contraction, a highly organized structure composed of overlapping protein filaments:
- Actin filaments: Thin filaments primarily composed of actin, tropomyosin, and troponin. These are anchored at the Z-lines.
- Myosin filaments: Thick filaments composed of myosin molecules with globular heads that project outwards. These are situated within the A-band.
Within the sarcomere, distinct regions are identified:
- A-band (Anisotropic band): The dark band containing the entire length of the myosin filaments, including overlapping regions with actin filaments. The A-band does not shorten during contraction.
- I-band (Isotropic band): The light band containing only actin filaments. This is the region we'll focus on.
- H-zone: A lighter region within the A-band where actin and myosin filaments do not overlap.
- Z-line (Z-disc): A dense, protein-rich structure that separates adjacent sarcomeres and anchors the actin filaments.
The Sliding Filament Theory: The Mechanism of Muscle Contraction
The sliding filament theory explains muscle contraction as the sliding of actin filaments past myosin filaments, causing the sarcomere to shorten. This process doesn't involve a change in the length of the individual filaments themselves, but rather a change in their relative overlap.
The process is initiated by a nerve impulse that triggers the release of calcium ions (Ca²⁺) into the sarcoplasm. Ca²⁺ binds to troponin, causing a conformational change that exposes myosin-binding sites on the actin filaments.
Myosin heads, possessing ATPase activity, then bind to these exposed sites, forming cross-bridges. The power stroke, fueled by ATP hydrolysis, pulls the actin filaments towards the center of the sarcomere. This process repeats as long as Ca²⁺ and ATP are available, resulting in muscle contraction.
What Happens to the I Band During Contraction?
Now, we can address the central question: Does the I-band shorten during contraction?
The answer is yes. As the actin filaments slide past the myosin filaments during contraction, the I-band, which solely comprises actin filaments, shortens significantly. The H-zone, representing the area where only myosin filaments are present, also shortens, eventually disappearing at maximal contraction. However, the A-band, representing the entire length of the myosin filament, remains unchanged in length.
This shortening of the I-band provides direct evidence of the sliding filament mechanism. It visually demonstrates the relative movement of actin and myosin filaments during muscle contraction.
The Role of Myosin Heads and Cross-Bridges
The precise interaction between myosin heads and actin filaments is crucial to understanding the shortening of the I-band. Each myosin head undergoes a cyclical process:
- Attachment: The myosin head binds to an exposed actin binding site.
- Power stroke: ATP hydrolysis causes a conformational change in the myosin head, pulling the actin filament towards the center of the sarcomere.
- Detachment: ATP binds to the myosin head, causing it to detach from the actin filament.
- Reactivation: The myosin head is re-energized, ready to bind to another actin binding site further along the filament.
This continuous cycle of attachment, power stroke, detachment, and reactivation, coordinated across numerous myosin heads, generates the force necessary for muscle contraction and the resulting shortening of the I-band.
Factors Affecting I-Band Shortening
Several factors influence the extent of I-band shortening during contraction:
- The number of active cross-bridges: A higher number of active cross-bridges results in greater force production and a more significant shortening of the I-band.
- The length of the sarcomere before contraction: The initial length of the sarcomere influences the optimal overlap between actin and myosin filaments, affecting the extent of potential shortening. There is an optimal length for maximal force generation. Beyond this optimal length, less overlap occurs and force production decreases.
- The availability of ATP: ATP hydrolysis is essential for the power stroke. ATP depletion leads to muscle fatigue and a reduced ability to shorten the I-band.
- Calcium ion concentration: Ca²⁺ ions are crucial for initiating the cross-bridge cycle. Lower Ca²⁺ levels result in weaker contractions and less I-band shortening.
Distinguishing I-band Shortening from Other Sarcomere Changes
It is important to note that while the I-band shortens significantly, the A-band remains constant. This is a critical distinction in understanding the sliding filament mechanism. The H-zone, like the I-band, also shortens and can even disappear entirely during maximal contraction. These specific changes help differentiate the sliding filament mechanism from other potential modes of muscle contraction.
Clinical Implications and Further Research
Understanding the changes within the sarcomere, particularly the behavior of the I-band during contraction, has significant implications in various fields:
- Muscle physiology: This knowledge forms the foundation for understanding muscle function in health and disease.
- Sports medicine: Optimizing training strategies and rehabilitation programs require a deep understanding of muscle mechanics.
- Cardiology: Heart muscle contraction shares similarities with skeletal muscle, and knowledge of sarcomere dynamics is essential in understanding cardiac function.
- Neuromuscular disorders: Many neuromuscular diseases affect the integrity of the sarcomere, leading to impaired muscle function. Understanding the microscopic changes provides insights into the pathophysiology of these conditions.
Ongoing research continues to refine our understanding of the intricacies of muscle contraction. Advanced imaging techniques allow for detailed visualization of sarcomere dynamics during contraction, providing more precise measurements of I-band shortening and other structural changes.
Conclusion: I-Band Shortening as Evidence of the Sliding Filament Theory
The shortening of the I-band during muscle contraction is a pivotal observation supporting the sliding filament theory. It clearly demonstrates the relative movement of actin and myosin filaments, the fundamental mechanism of force generation in skeletal muscles. By understanding this process, we gain critical insight into muscle physiology, impacting various fields from sports science to medical research. Further research will continue to unveil the intricacies of this complex process, improving our understanding of both normal muscle function and the pathologies that affect it. The meticulous study of the I-band’s behavior remains a cornerstone in unraveling the mysteries of muscle contraction and the remarkable ability of our bodies to generate movement.
Latest Posts
Latest Posts
-
The Apc Can Be Defined As The Fraction Of A
Mar 19, 2025
-
You Throw Away The Outside And Cook The Inside
Mar 19, 2025
-
What Is The Distance Between The Sun And Saturn
Mar 19, 2025
-
Why Are Producers Important To The Ecosystem
Mar 19, 2025
-
Example Of A Small Scale Map
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Does The I Band Shorten During Contraction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
