Why Is The Boiling Of Water A Physical Change
News Leon
Mar 23, 2025 · 5 min read
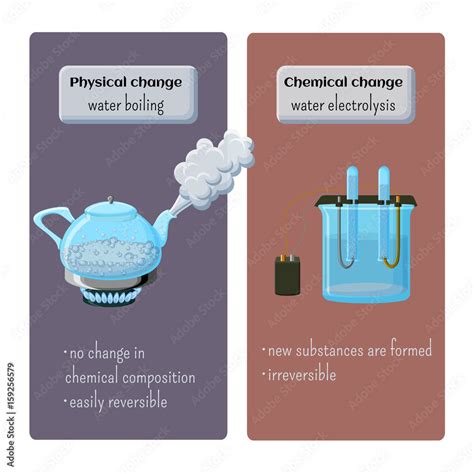
Table of Contents
Why is the Boiling of Water a Physical Change? Understanding Phase Transitions
Boiling water is a quintessential example of a physical change, a transformation that alters the form or appearance of a substance but not its chemical composition. Understanding why this seemingly simple process is classified as a physical change requires a deep dive into the nature of matter and the phases of water. This comprehensive guide will explore the microscopic and macroscopic aspects of boiling water, definitively establishing its classification as a physical change and debunking common misconceptions.
The Microscopic Dance of Water Molecules
At the heart of understanding boiling lies the behavior of water molecules (H₂O). These molecules are constantly in motion, their kinetic energy determining the state of the water. In a solid (ice), the molecules are tightly packed in a rigid structure, vibrating in place. As heat is added, the kinetic energy increases, causing the molecules to vibrate more vigorously. This ultimately leads to the melting point, where the structure breaks down and the water transitions into a liquid.
In liquid water, the molecules are closer together than in gas, but they have more freedom of movement. They slide past each other, allowing for fluidity. However, they still exert attractive forces (hydrogen bonds) upon each other, maintaining a degree of cohesion.
As more heat is supplied, the kinetic energy of the water molecules continues to rise. This leads to the boiling point, a critical temperature where the kinetic energy overcomes the intermolecular forces holding the molecules together in the liquid state. At this point, a significant transformation occurs.
The Escape Velocity of Water Molecules
At the boiling point, water molecules gain enough kinetic energy to overcome the attractive forces of their neighbors and escape into the gaseous phase (steam). They transition from a relatively ordered, closely-packed arrangement to a disordered, widely dispersed state. This is a key characteristic of a physical change—the molecules themselves remain intact, even though their arrangement and state change dramatically.
Crucially, the chemical bonds within each individual water molecule (the covalent bonds between the oxygen and hydrogen atoms) remain unbroken throughout the boiling process. This is the defining feature differentiating a physical change from a chemical change. In a chemical change, the chemical bonds within the molecules would be broken or reformed, resulting in the formation of new substances.
Macroscopic Observations of a Physical Change
The macroscopic changes observed during boiling further solidify its classification as a physical change:
-
Change in State: The most obvious change is the transition from liquid to gas. This is a physical change because it involves a change in physical properties (density, viscosity, etc.) rather than a change in chemical composition.
-
Reversibility: Boiling is a reversible process. If the steam is cooled, it will condense back into liquid water, demonstrating the unchanged chemical nature of the substance. This reversibility is a hallmark of physical changes. Chemical changes often produce irreversible transformations.
-
No New Substance Formed: No new chemical compound or element is created during boiling. The steam produced is still water (H₂O), albeit in a different physical state. This lack of new substance formation is another key indicator of a physical change.
-
Energy Change (Physical, Not Chemical): The energy absorbed during boiling is used to overcome the intermolecular forces holding the water molecules together, allowing them to transition into the gas phase. This energy change is a physical phenomenon, not a chemical reaction. Chemical reactions involve energy changes associated with the breaking and forming of chemical bonds.
Debunking Common Misconceptions
Despite the clear evidence, some misconceptions persist about the boiling of water:
-
Myth: Boiling water is a chemical change because heat is involved. Reality: Heat is involved in many physical changes. Heat supplies the energy needed to change the state of matter, not to break or form chemical bonds.
-
Myth: Steam is different from water because it looks and behaves differently. Reality: Steam is still water (H₂O). The differences in appearance and behavior are due to the change in state, not a change in chemical composition.
-
Myth: Bubbles forming during boiling indicate a chemical reaction. Reality: Bubbles are formed by water vapor (steam) rising to the surface. These bubbles are simply water molecules in the gaseous state, escaping the liquid phase.
Comparing Physical and Chemical Changes: A Clear Distinction
Let's highlight the fundamental differences between physical and chemical changes to solidify the understanding of boiling water as a physical change.
| Feature | Physical Change (Boiling Water) | Chemical Change |
|---|---|---|
| Chemical Composition | Remains the same (H₂O) | Changes (new substances formed) |
| Bonds | Intact | Broken and/or formed |
| Reversibility | Usually reversible | Usually irreversible |
| Energy Change | Associated with phase transitions | Associated with bond breaking/formation |
| Examples | Melting, freezing, boiling, evaporation, condensation | Burning, rusting, digestion, cooking |
Beyond Boiling: Other Phase Transitions
The boiling of water is just one example of a phase transition, a physical change that involves a change in the state of matter. Other examples include:
- Melting: The transition from solid to liquid.
- Freezing: The transition from liquid to solid.
- Evaporation: The transition from liquid to gas below the boiling point.
- Condensation: The transition from gas to liquid.
- Sublimation: The transition from solid directly to gas (e.g., dry ice).
- Deposition: The transition from gas directly to solid (e.g., frost formation).
All of these phase transitions share the common characteristic of involving a change in physical state without altering the chemical composition of the substance.
Conclusion: Boiling Water – A Clear Case of a Physical Change
In conclusion, the boiling of water is unequivocally a physical change. While the macroscopic appearance of water changes dramatically, the microscopic chemical composition remains identical. The molecules simply rearrange themselves and move with greater energy, transitioning from the liquid to the gaseous phase. The reversibility of the process, the lack of new substance formation, and the energy changes associated with phase transitions further solidify this classification. Understanding this fundamental principle lays the groundwork for grasping more complex concepts in chemistry and physics. The seemingly simple act of boiling water offers a powerful illustration of the profound interplay between energy, matter, and phase transitions.
Latest Posts
Latest Posts
-
Definition Of Order Of A Reaction
Mar 25, 2025
-
Distilled Water Does Not Conduct A Current
Mar 25, 2025
-
A Projectile Is Fired Horizontally From A Gun
Mar 25, 2025
-
Why Europe Is Called The Peninsula Of Peninsulas
Mar 25, 2025
-
Which Of The Following Is A Mineralocorticoid
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Why Is The Boiling Of Water A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
