Definition Of Order Of A Reaction
News Leon
Mar 25, 2025 · 6 min read
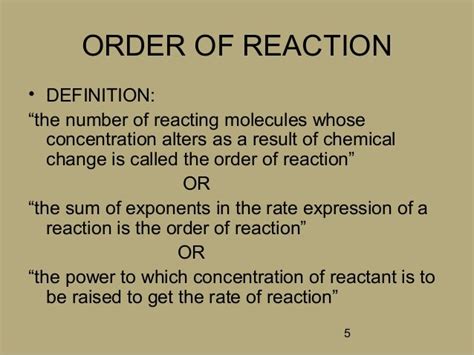
Table of Contents
Understanding the Order of a Reaction: A Comprehensive Guide
The order of a reaction is a fundamental concept in chemical kinetics, describing how the rate of a reaction changes with the concentration of reactants. Understanding this concept is crucial for predicting reaction behavior, designing chemical processes, and interpreting experimental data. This comprehensive guide delves deep into the definition, determination, and significance of reaction order, providing a detailed explanation suitable for students and professionals alike.
What is the Order of a Reaction?
The order of a reaction with respect to a specific reactant is defined as the power to which the concentration of that reactant is raised in the rate law. The overall order of a reaction is the sum of the orders with respect to each reactant. It's vital to remember that reaction order is an experimental quantity, not something derived directly from the stoichiometric coefficients of the balanced chemical equation. The reaction mechanism determines the rate law, which in turn dictates the reaction order.
Consider a generic reaction:
aA + bB → cC + dD
The rate law is generally expressed as:
Rate = k[A]^m[B]^n
Where:
- k is the rate constant (temperature-dependent).
- [A] and [B] represent the concentrations of reactants A and B.
- m and n are the orders of the reaction with respect to reactants A and B, respectively. These are typically integers (0, 1, 2, etc.), but can also be fractional or negative in some complex reactions.
- m + n represents the overall order of the reaction.
Examples of Different Reaction Orders:
-
Zero-order reaction (m+n = 0): The rate is independent of the concentration of reactants. This often occurs when the reaction is surface-area limited or when a catalyst is saturated. The rate law is simply: Rate = k.
-
First-order reaction (m+n = 1): The rate is directly proportional to the concentration of one reactant. A classic example is radioactive decay. The rate law could be: Rate = k[A] or Rate = k[B], depending on whether the reaction is first-order with respect to A or B.
-
Second-order reaction (m+n = 2): The rate is proportional to the square of the concentration of one reactant or the product of the concentrations of two reactants. Many bimolecular reactions fall into this category. Examples of rate laws include: Rate = k[A]^2 or Rate = k[A][B].
-
Higher-order reactions (m+n > 2): These reactions are less common and involve higher powers of reactant concentrations in the rate law. Their kinetics can be significantly more complex.
-
Fractional-order reactions: In certain complex reactions, the reaction order can be a fraction, indicating a more intricate mechanism involving multiple steps.
-
Negative-order reactions: A negative order with respect to a reactant indicates that an increase in the concentration of that reactant decreases the reaction rate. This often arises in reactions where a reactant inhibits the reaction or when an intermediate product acts as an inhibitor.
Determining the Order of a Reaction: Experimental Methods
Determining the reaction order experimentally is crucial for understanding the reaction mechanism and predicting its behavior under different conditions. Several methods are commonly employed:
1. Method of Initial Rates:
This is a widely used method that involves measuring the initial rate of the reaction at different initial concentrations of reactants. By comparing the rates at different concentrations, the order with respect to each reactant can be determined.
Steps:
- Conduct experiments: Perform multiple experiments, varying the initial concentration of one reactant while keeping others constant. Record the initial rate for each experiment.
- Compare rates: Analyze the data to see how the initial rate changes with changes in concentration.
- Determine the order: If doubling the concentration of a reactant doubles the rate, the reaction is first-order with respect to that reactant. If doubling the concentration quadruples the rate, it's second-order, and so on.
2. Integrated Rate Laws:
Each reaction order has a corresponding integrated rate law, which relates the concentration of a reactant to time. By plotting the appropriate function of concentration versus time, the order and rate constant can be determined from the slope and intercept of the resulting straight line.
- Zero-order: [A] = -kt + [A]₀ (plot [A] vs. t; slope = -k)
- First-order: ln[A] = -kt + ln[A]₀ (plot ln[A] vs. t; slope = -k)
- Second-order: 1/[A] = kt + 1/[A]₀ (plot 1/[A] vs. t; slope = k)
3. Half-life Method:
The half-life (t₁/₂) of a reaction is the time required for the concentration of a reactant to decrease to half its initial value. The half-life is dependent on the order of the reaction and the rate constant. Analyzing how the half-life changes with initial concentration can help determine the reaction order.
- First-order: t₁/₂ = 0.693/k (independent of initial concentration)
- Second-order: t₁/₂ = 1/(k[A]₀) (inversely proportional to initial concentration)
Significance of Reaction Order
Understanding the order of a reaction has several important implications:
- Predicting reaction rates: Knowing the rate law allows us to predict the rate of a reaction under various conditions of reactant concentration.
- Reaction mechanism elucidation: The reaction order provides valuable insights into the reaction mechanism. The rate-determining step often dictates the overall reaction order.
- Reactor design: The reaction order is a critical parameter in designing chemical reactors, optimizing reaction conditions, and controlling product yield.
- Pharmacokinetics and drug delivery: In pharmacology, reaction order is crucial for understanding drug metabolism and designing effective drug delivery systems.
- Environmental chemistry: Reaction order plays a significant role in understanding the rates of environmental processes such as pollutant degradation and atmospheric reactions.
Complexities and Exceptions
While the concepts of reaction order are relatively straightforward, several complexities can arise:
- Non-integer orders: Fractional or negative orders often indicate complex reaction mechanisms involving multiple steps and intermediates.
- Temperature dependence: The rate constant (k) is temperature-dependent, following the Arrhenius equation. Changes in temperature can affect the reaction rate and may influence the apparent order under certain conditions.
- Side reactions and catalysts: The presence of side reactions or catalysts can significantly impact the observed reaction order.
- Steady-state approximation: In complex reaction mechanisms, the steady-state approximation can be used to simplify the rate law and derive a pseudo-order rate equation.
Conclusion
The order of a reaction is a fundamental concept in chemical kinetics, describing the relationship between reactant concentrations and reaction rate. Its determination through experimental methods such as the method of initial rates, integrated rate laws, or half-life analysis is essential for understanding reaction mechanisms and predicting reaction behavior. Understanding reaction order is crucial across numerous scientific and engineering disciplines, from chemical reactor design to drug development and environmental modeling. While seemingly simple in definition, the intricacies of determining and interpreting reaction order highlight the complexity and elegance of chemical kinetics. A thorough grasp of this concept lays a solid foundation for more advanced studies in reaction dynamics and chemical processes.
Latest Posts
Latest Posts
-
The Standard Unit Of Mass Is
Mar 26, 2025
-
What Is The Oxidation Number Of Cr In K2cr2o7
Mar 26, 2025
-
Which Of The Following Has Kinetic Energy
Mar 26, 2025
-
Which Of The Following Is Not An Intrusive Igneous Body
Mar 26, 2025
-
Which Of The Following Is An Extensive Property Of Matter
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Definition Of Order Of A Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
