What Is The Oxidation Number Of Cr In K2cr2o7
News Leon
Mar 26, 2025 · 5 min read
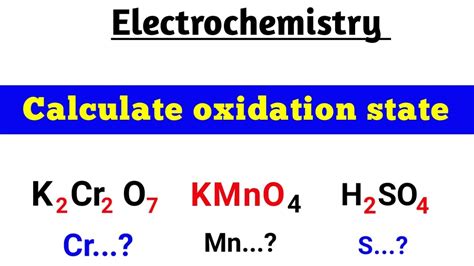
Table of Contents
What is the Oxidation Number of Cr in K₂Cr₂O₇? A Comprehensive Guide
Determining the oxidation number of chromium (Cr) in potassium dichromate (K₂Cr₂O₇) is a fundamental concept in chemistry, crucial for understanding redox reactions and balancing chemical equations. This comprehensive guide will not only answer this question but also delve into the underlying principles, providing a thorough understanding of oxidation numbers and their application.
Understanding Oxidation Numbers
Oxidation numbers, also known as oxidation states, represent the hypothetical charge an atom would have if all bonds to atoms of different elements were completely ionic. They are a bookkeeping tool, incredibly useful in predicting the reactivity of elements and compounds. While not a true charge, oxidation numbers help us track electron transfer during chemical reactions.
Key Rules for Assigning Oxidation Numbers:
- Rule 1: The oxidation number of an element in its free (uncombined) state is always 0. For example, the oxidation number of O₂ is 0, and the oxidation number of Fe in a piece of iron is 0.
- Rule 2: The oxidation number of a monatomic ion is equal to its charge. For instance, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
- Rule 3: The oxidation number of hydrogen is +1, except in metal hydrides where it is -1. Water (H₂O) has hydrogen with an oxidation number of +1, while in sodium hydride (NaH), hydrogen has an oxidation number of -1.
- Rule 4: The oxidation number of oxygen is usually -2, except in peroxides (like H₂O₂) where it is -1 and in superoxides (like KO₂) where it is -1/2.
- Rule 5: The sum of the oxidation numbers of all atoms in a neutral molecule is 0.
- Rule 6: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Determining the Oxidation Number of Cr in K₂Cr₂O₇
Now, let's apply these rules to determine the oxidation number of chromium (Cr) in potassium dichromate (K₂Cr₂O₇).
Step-by-step calculation:
-
Identify the known oxidation numbers: Potassium (K) is an alkali metal, always having an oxidation number of +1. Oxygen (O) usually has an oxidation number of -2 (as per Rule 4).
-
Assign variables: Let's denote the oxidation number of chromium (Cr) as 'x'.
-
Apply Rule 5 (sum of oxidation numbers in a neutral molecule is 0): In K₂Cr₂O₇, we have:
(2 × oxidation number of K) + (2 × oxidation number of Cr) + (7 × oxidation number of O) = 0
-
Substitute the known oxidation numbers:
(2 × (+1)) + (2 × x) + (7 × (-2)) = 0
-
Solve for x:
2 + 2x - 14 = 0 2x = 12 x = +6
Therefore, the oxidation number of Cr in K₂Cr₂O₇ is +6.
The Significance of the +6 Oxidation State of Chromium
The +6 oxidation state of chromium is particularly important because it signifies a highly oxidized form of the element. This high oxidation state makes Cr(VI) compounds potent oxidizing agents. This means they readily accept electrons from other substances, causing them to be reduced. Potassium dichromate itself is a common oxidizing agent in various chemical reactions and applications.
Applications of K₂Cr₂O₇ and Cr(VI) Compounds:
- Organic Chemistry: K₂Cr₂O₇ is used as an oxidizing agent in organic chemistry for various transformations like oxidation of alcohols to aldehydes or ketones.
- Analytical Chemistry: It finds application in titrations to determine the concentration of reducing agents.
- Leather Tanning: Historically, Cr(VI) compounds were used extensively in the leather tanning industry, though this use is declining due to environmental concerns.
- Metal Finishing: Chromium plating utilizes Cr(VI) compounds, although newer, less toxic methods are being developed.
Caution: Toxicity of Cr(VI) Compounds
It is crucial to emphasize that chromium(VI) compounds, including potassium dichromate, are highly toxic and carcinogenic. They pose significant health risks through inhalation, ingestion, or skin contact. Always handle these compounds with extreme care, using appropriate safety measures, including gloves, eye protection, and a well-ventilated area. Proper disposal according to local regulations is mandatory.
Further Exploration: Other Chromium Oxidation States
Chromium exhibits a wide range of oxidation states, including +2, +3, and +6, each having unique chemical properties and reactivities.
- Cr(II) (Chromium(II)): Often found in compounds like chromium(II) chloride (CrCl₂), it is a reducing agent and easily oxidized to higher oxidation states.
- Cr(III) (Chromium(III)): Relatively stable and common, seen in compounds like chromium(III) oxide (Cr₂O₃) and chromium(III) sulfate (Cr₂(SO₄)₃). It is less toxic than Cr(VI).
- Cr(VI) (Chromium(VI)): As discussed above, this oxidation state is characterized by strong oxidizing properties and significant toxicity.
Redox Reactions and Oxidation Numbers
Oxidation numbers are essential in understanding redox (reduction-oxidation) reactions. Redox reactions involve the transfer of electrons between species. Oxidation involves the loss of electrons (increase in oxidation number), while reduction involves the gain of electrons (decrease in oxidation number).
In the context of K₂Cr₂O₇, the chromium in the +6 oxidation state can act as an oxidizing agent, gaining electrons and being reduced to a lower oxidation state during a redox reaction. For example, in the reaction with ethanol, the chromium(VI) is reduced to chromium(III) while the ethanol is oxidized to acetic acid.
Conclusion: Mastering Oxidation Numbers
The determination of the oxidation number of chromium in K₂Cr₂O₇, as +6, underscores the importance of understanding and applying the rules for assigning oxidation numbers. This seemingly simple calculation underpins a deeper understanding of redox chemistry, the properties of transition metals, and the significance of oxidation states in determining the reactivity and toxicity of chemical compounds. Remember always to prioritize safety when handling chemicals, especially those with toxic properties like potassium dichromate. This comprehensive exploration provides a solid foundation for further study in inorganic chemistry and related fields. Understanding oxidation numbers is fundamental to advancing your knowledge in chemistry and its applications.
Latest Posts
Latest Posts
-
What Is The Angular Speed Of Earth
Mar 27, 2025
-
This Is The Measure Of Disorder In A System
Mar 27, 2025
-
A Group Of 8 Bits Is Known As A
Mar 27, 2025
-
What Is The Common Multiple Of 4 And 9
Mar 27, 2025
-
What Is A Negative Ion Called
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Is The Oxidation Number Of Cr In K2cr2o7 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
