What Is A Negative Ion Called
News Leon
Mar 27, 2025 · 6 min read
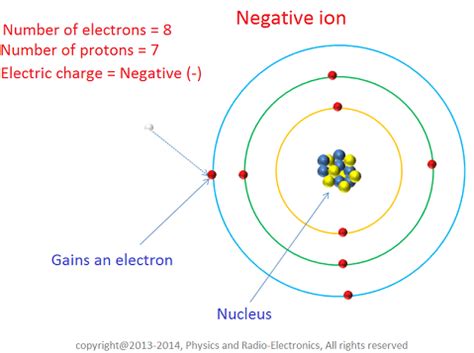
Table of Contents
What is a Negative Ion Called? Exploring Anions and Their Significance
The simple question, "What is a negative ion called?", unveils a fascinating world of chemistry and physics. While the everyday term "negative ion" is perfectly acceptable in casual conversation, the formal scientific term is anion. This article delves deep into the nature of anions, exploring their properties, formation, significance in various fields, and addressing common misconceptions.
Understanding Ions: The Foundation of Anions
Before diving into the specifics of anions, it's crucial to understand the broader concept of ions. Ions are atoms or molecules that carry an electric charge. This charge arises from an imbalance in the number of protons (positively charged particles) and electrons (negatively charged particles).
- Cations: When an atom loses one or more electrons, it becomes positively charged and is called a cation. These are often formed by metals, which readily lose electrons.
- Anions: Conversely, when an atom gains one or more electrons, it becomes negatively charged and is termed an anion. These are commonly formed by nonmetals, which have a higher affinity for electrons.
The charge of an ion is denoted by a superscript following the chemical symbol. For example, a chloride ion (Cl⁻) has a single negative charge, while an oxide ion (O²⁻) has a double negative charge. The magnitude of the charge indicates the number of electrons gained or lost.
The Formation of Anions: The Electron's Role
The formation of anions hinges on the concept of electron affinity. Electron affinity refers to the energy change that occurs when an atom gains an electron. Atoms with a high electron affinity readily accept electrons, forming stable anions. This stability often arises from achieving a full outer electron shell (octet rule), a configuration that minimizes energy and enhances stability.
Several factors influence an atom's electron affinity:
- Atomic size: Smaller atoms generally have higher electron affinities because the incoming electron experiences a stronger attraction to the nucleus.
- Nuclear charge: A higher nuclear charge increases the attractive force on the incoming electron, favoring anion formation.
- Electron shielding: Inner electrons shield the outer electrons from the full nuclear charge. Increased shielding reduces the attraction for an incoming electron.
The process of anion formation is often exothermic, meaning it releases energy. This energy release contributes to the stability of the anion.
Types and Examples of Anions: A Diverse Group
Anions encompass a wide variety of chemical species, exhibiting diverse properties and playing critical roles in numerous processes. Here are some key examples categorized for clarity:
Monatomic Anions: Simple Negative Ions
These are formed from single atoms gaining electrons. Common examples include:
- Halide ions: Fluoride (F⁻), chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻). These are crucial in biological systems and industrial processes.
- Chalcogenide ions: Oxide (O²⁻), sulfide (S²⁻), selenide (Se²⁻), and telluride (Te²⁻). These form the basis of many minerals and compounds.
- Nitride ion: Nitride (N³⁻), found in certain compounds like nitrides of transition metals.
Polyatomic Anions: Multiple Atoms United in Negativity
These anions are composed of two or more atoms covalently bonded and carrying a net negative charge. This group displays remarkable diversity and functional complexity:
-
Oxyanions: These contain oxygen atoms bonded to a central atom. Examples include:
- Sulfate (SO₄²⁻): Crucial in many biological processes and industrial applications.
- Nitrate (NO₃⁻): A key component of fertilizers and explosives.
- Phosphate (PO₄³⁻): Essential in biological systems, particularly in DNA and energy transfer.
- Carbonate (CO₃²⁻): A major component of limestone and other carbonates.
-
Other polyatomic anions:
- Hydroxide (OH⁻): A crucial component of bases and involved in many chemical reactions.
- Acetate (CH₃COO⁻): A common organic anion found in vinegar.
- Cyanide (CN⁻): A highly toxic anion.
The Significance of Anions Across Disciplines
Anions play pivotal roles in a wide range of scientific fields:
Biology and Medicine: Essential for Life
Anions are fundamental to biological processes. Many essential biological molecules are anions, including:
- Phosphate in ATP: Adenosine triphosphate (ATP) is the primary energy currency of cells, containing phosphate anions.
- Amino acids: Many amino acids, the building blocks of proteins, exist as anions at physiological pH.
- DNA and RNA: These genetic materials contain phosphate anions in their backbones.
- Electrolyte balance: Anions like chloride and bicarbonate contribute to the electrolyte balance in the body, crucial for proper nerve and muscle function.
Disruptions in anion balance can lead to various health issues, highlighting their importance in maintaining homeostasis.
Chemistry and Material Science: Building Blocks of Matter
Anions are the cornerstone of numerous chemical compounds and materials. Their properties significantly influence the characteristics of these substances:
- Salt formation: Anions combine with cations to form ionic compounds, commonly known as salts. These salts exhibit a vast range of properties depending on the specific anion and cation involved.
- Material properties: The type of anion present in a material greatly influences its physical properties, such as hardness, conductivity, and melting point.
- Catalysis: Certain anions act as catalysts, accelerating chemical reactions.
Environmental Science: Shaping Our World
Anions play crucial roles in environmental processes:
- Water chemistry: Anions like sulfate, nitrate, and phosphate are significant components of water quality. Elevated levels of these anions can lead to water pollution and eutrophication.
- Soil chemistry: Anions influence soil fertility and plant growth.
- Atmospheric chemistry: Anions are involved in atmospheric processes, including the formation of acid rain.
Understanding anion behavior in the environment is essential for developing effective environmental management strategies.
Misconceptions about Negative Ions: Separating Fact from Fiction
While the term "negative ion" is widely used, some misconceptions surround its meaning and implications. Let's address a few:
Myth 1: Negative ions are always beneficial for health.
While some research suggests certain negative ions, like those produced by natural phenomena like waterfalls, may have minor positive effects on mood and well-being, this is not universally accepted and requires further rigorous study. Claims of significant health benefits often lack strong scientific evidence. The concentration and type of anion are important factors.
Myth 2: All negative ions are the same.
This is incorrect. Anions vary greatly in their chemical properties and biological effects. A chloride ion (Cl⁻) is vastly different from a phosphate ion (PO₄³⁻). It is misleading to broadly categorize all anions as having the same impacts.
Myth 3: "Negative ion generators" significantly improve air quality.
These devices often claim to release beneficial negative ions into the air. However, the actual impact on air quality is minimal at best and often lacks robust scientific backing. The concentration of ions produced is often negligible compared to natural sources. Proper ventilation and filtration remain the most effective means of improving indoor air quality.
Conclusion: Anions – Tiny Particles, Immense Significance
In conclusion, the term "negative ion" is commonly used, but the formal scientific term is anion. These negatively charged species are far from simple; they are fundamental building blocks of matter, essential for life, and play pivotal roles in various scientific disciplines. Understanding their properties, formation, and significance is crucial for advancements in numerous fields, from medicine and materials science to environmental management. It's important to approach claims about their purported health benefits with critical thinking and a healthy dose of skepticism, focusing on robust scientific evidence.
Latest Posts
Latest Posts
-
Which Of The Following Measurement Is More Accurate
Mar 30, 2025
-
What Is The Conjugate Base Of H2so4
Mar 30, 2025
-
Find The Measure Of Each Lettered Angle In The Figure
Mar 30, 2025
-
Which Issue Does Terrace Farming Help Solve
Mar 30, 2025
-
Which Of The Following Sequences Are Geometric
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is A Negative Ion Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
