Distilled Water Does Not Conduct A Current..
News Leon
Mar 25, 2025 · 6 min read
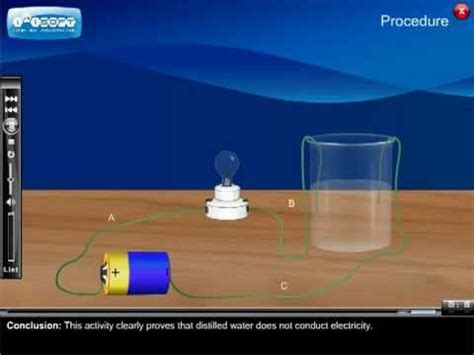
Table of Contents
Distilled Water: A Non-Conductor of Electricity
Distilled water, unlike tap water or other naturally occurring water sources, does not readily conduct electricity. This seemingly simple fact underpins numerous scientific principles and has significant implications across various industries. Understanding why distilled water behaves this way, and the nuances surrounding its electrical conductivity, is crucial for appreciating its applications and limitations.
The Role of Ions in Electrical Conductivity
The ability of a substance to conduct electricity is directly related to its ability to allow the movement of charged particles, known as ions. These ions are atoms or molecules that have gained or lost electrons, resulting in a net positive or negative charge. In the case of water, the presence of dissolved ions is the key determinant of its conductivity.
Ions in Tap Water
Tap water, unlike distilled water, contains a variety of dissolved minerals and impurities, including salts, metals, and other contaminants. These substances dissociate into ions when dissolved in water. For instance, table salt (sodium chloride, NaCl) dissolves into sodium ions (Na⁺) and chloride ions (Cl⁻). These freely moving ions act as charge carriers, allowing electricity to flow through the water. The higher the concentration of dissolved ions, the greater the conductivity of the water.
The Purity of Distilled Water
Distillation is a purification process that removes virtually all dissolved ions and other impurities from water. The process involves boiling water and then condensing the steam, leaving behind the dissolved solids. This results in water that is exceptionally pure, with a significantly reduced concentration of ions. Because the number of free-moving ions in distilled water is minimal, it offers significantly less resistance to the flow of electricity. It's important to remember that pure distilled water itself does not contain significant ions to conduct electricity.
Why Distilled Water Doesn't Conduct (Well)
The lack of conductivity in distilled water is a direct consequence of its purity. With minimal dissolved ions, there are few charge carriers available to facilitate the movement of electric current. While theoretically pure water contains a tiny number of self-ionized water molecules (H₃O⁺ and OH⁻), their concentration is extremely low (around 10⁻⁷ moles per liter at 25°C), making their contribution to conductivity negligible.
The Concept of Self-Ionization
Water molecules themselves can undergo a process called self-ionization, where a water molecule spontaneously donates a proton (H⁺) to another water molecule, forming a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻). This process is an equilibrium reaction, meaning that it occurs in both directions simultaneously. However, the equilibrium constant for this reaction is extremely small, meaning that the concentration of H₃O⁺ and OH⁻ ions is very low.
Impurities Affect Conductivity
Even trace amounts of impurities can significantly affect the conductivity of distilled water. Exposure to air, for example, can introduce carbon dioxide (CO₂), which dissolves to form carbonic acid (H₂CO₃), slightly increasing the conductivity. Similarly, contact with container materials can leach ions into the water, altering its electrical properties. This is why storing distilled water in appropriate containers (such as glass) is important to maintain its low conductivity.
Measuring the Conductivity of Water
The conductivity of water is measured using a conductivity meter or a conductometer. This device measures the ability of water to conduct an electrical current, typically expressed in microsiemens per centimeter (µS/cm) or millisiemens per centimeter (mS/cm). Distilled water typically exhibits very low conductivity values, usually less than 5 µS/cm. Higher values indicate the presence of more dissolved ions and impurities.
Factors Affecting Conductivity Measurements
Several factors can affect the accuracy of conductivity measurements, including:
- Temperature: Conductivity increases with temperature.
- Calibration: Regular calibration of the conductivity meter is essential for accurate readings.
- Sample Handling: Careful handling of the water sample to avoid contamination is crucial.
Applications Leveraging Distilled Water's Non-Conductivity
The low conductivity of distilled water makes it ideal for several applications where the presence of ions would be detrimental:
Batteries and Electronics
Distilled water is often used in lead-acid batteries as an electrolyte because its lack of impurities helps to prevent the short-circuiting of the battery. This ensures optimal battery performance and lifespan. Similarly, distilled water is crucial in certain electronic components and processes where the presence of dissolved minerals could lead to corrosion and malfunction.
Chemical Experiments and Analysis
In many chemical experiments and analyses, the purity of distilled water is essential. The absence of interfering ions ensures that experimental results are not affected by the presence of impurities. This is critical in various applications like analytical chemistry, biochemistry, and other precise scientific studies.
Automotive Applications
Distilled water is commonly used in car batteries and cooling systems. As discussed before, in batteries, it helps maintain the electrolyte's conductivity and prevents short circuits. In cooling systems, it prevents the buildup of mineral deposits and corrosion, which can damage engine components.
Medical and Pharmaceutical Applications
The purity of distilled water is critical in various medical and pharmaceutical applications. It is used in the preparation of intravenous solutions, pharmaceuticals, and other sterile preparations where the presence of contaminants could be harmful. Strict standards regarding the purity of water are vital in these contexts, and distilled water often satisfies these stringent requirements.
Steam Ironing
The use of distilled water in steam irons prevents mineral buildup and scaling that can reduce efficiency and damage the appliance. The absence of ions prevents the formation of limescale, prolonging the life of the steam iron.
Distinguishing Distilled Water from Other Types of Purified Water
It is important to note that distilled water is not the only type of purified water. Other purification methods, such as reverse osmosis and deionization, also produce water with low conductivity. However, these methods differ in the type of impurities they remove.
Reverse Osmosis (RO) Water
Reverse osmosis filters water through a semipermeable membrane, removing a wide range of dissolved solids, including salts and other contaminants. While generally having lower conductivity than tap water, RO water may still contain some dissolved ions.
Deionized (DI) Water
Deionization removes ions from water by passing it through ion-exchange resins. This method produces water with exceptionally low conductivity, often comparable to or even lower than that of distilled water. However, it doesn't necessarily remove all non-ionic impurities, such as bacteria or organic matter.
The Importance of Purity and Context
The statement that "distilled water does not conduct a current" is a simplification. While pure distilled water exhibits very low conductivity, trace impurities or variations in the preparation process can affect its electrical properties. The key takeaway is that the low conductivity of distilled water is a direct result of its minimal ionic content. This property makes it invaluable in various applications where the absence of ions is critical for performance, safety, and accuracy. Understanding these nuances is important for correctly applying distilled water in a multitude of contexts, scientific or otherwise. The context always requires an understanding of the desired level of purity and what level of conductivity is acceptable for a particular use case.
Latest Posts
Latest Posts
-
The Most Abundant Metal In The Earths Crust Is
Mar 26, 2025
-
Of The Following Which Atom Has The Largest Atomic Radius
Mar 26, 2025
-
How Many Valence Electrons Does Alkali Metals Have
Mar 26, 2025
-
What Is The Conjugate Base Of H2po4
Mar 26, 2025
-
The Standard Unit Of Mass Is
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Distilled Water Does Not Conduct A Current.. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
