Of The Following Which Atom Has The Largest Atomic Radius
News Leon
Mar 26, 2025 · 5 min read
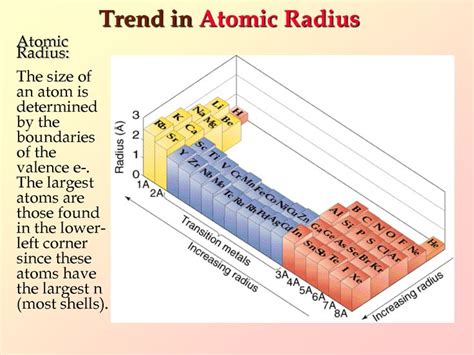
Table of Contents
Of the Following, Which Atom Has the Largest Atomic Radius? Understanding Atomic Size Trends
Determining which atom possesses the largest atomic radius requires a nuanced understanding of atomic structure and periodic trends. While a simple answer might seem sufficient, a deeper dive into the underlying principles is crucial for truly grasping the concept. This comprehensive article will explore the factors influencing atomic radius, delve into specific examples, and equip you with the knowledge to predict relative atomic sizes across the periodic table.
Understanding Atomic Radius
The atomic radius isn't a precisely defined measurement like the length of a table. Instead, it's a representation of the average distance between the nucleus of an atom and its outermost electron. Think of it as the effective size of an atom, though it's important to remember that electron clouds are probabilistic, not sharply defined boundaries.
Several factors significantly affect the atomic radius:
1. Number of Protons (Nuclear Charge): A greater number of protons in the nucleus exerts a stronger attractive force on the electrons, pulling them closer and resulting in a smaller atomic radius. This is a dominant trend across periods (rows) of the periodic table.
2. Number of Electron Shells (Energy Levels): As you move down a group (column) on the periodic table, you add more electron shells. These additional shells increase the distance between the nucleus and the outermost electrons, leading to a larger atomic radius.
3. Shielding Effect: Inner electrons partially shield the outermost electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge felt by the valence electrons, leading to a slightly larger atomic radius. The effectiveness of shielding isn't perfectly uniform and depends on the electron configuration.
4. Electron-Electron Repulsion: The repulsion between electrons in the same shell slightly increases the distance between them and the nucleus, subtly increasing the atomic radius. This effect becomes more significant with increasing numbers of electrons.
Periodic Trends and Atomic Radius
The periodic table is organized to reflect these trends. Understanding these trends allows for the prediction of relative atomic sizes without needing to know the exact numerical values:
Across a Period (Left to Right):
As you move from left to right across a period, the nuclear charge increases, but the electrons are added to the same shell. The increased nuclear charge outweighs the increased electron-electron repulsion, resulting in a decrease in atomic radius.
Down a Group (Top to Bottom):
As you move down a group, the number of electron shells increases significantly, leading to a substantial increase in atomic radius. The effect of adding another shell far outweighs the increased nuclear charge.
Comparing Atomic Radii: Examples
Let's consider some specific examples to illustrate these principles. Suppose we're comparing the atomic radii of several atoms: Lithium (Li), Sodium (Na), Potassium (K), and Fluorine (F).
-
Lithium (Li) vs. Fluorine (F): Both are in the same period (Period 2). Fluorine has a higher nuclear charge than Lithium. Therefore, Fluorine has a smaller atomic radius than Lithium.
-
Lithium (Li) vs. Sodium (Na): Both are in the same group (Group 1). Sodium has an additional electron shell compared to Lithium. Therefore, Sodium has a significantly larger atomic radius than Lithium.
-
Sodium (Na) vs. Potassium (K): Again, these are in the same group (Group 1). Potassium has another added electron shell compared to Sodium. Therefore, Potassium has a significantly larger atomic radius than Sodium.
This pattern holds true across the periodic table. Atoms lower down and further to the left generally have larger atomic radii.
Isoelectronic Series and Atomic Radius
An isoelectronic series is a group of atoms or ions that have the same number of electrons. In this case, the number of protons determines the atomic radius. An ion with more protons will have a smaller atomic radius than an ion with fewer protons, even if they have the same number of electrons.
For example, consider the isoelectronic series: O²⁻, F⁻, Ne, Na⁺, Mg²⁺. They all have 10 electrons. However, Mg²⁺ has the most protons, resulting in the strongest nuclear attraction and the smallest atomic radius. Conversely, O²⁻ has the fewest protons and the largest atomic radius within this series.
Factors Affecting Apparent Atomic Radius in Specific Cases
While the general trends are predictable, there are some exceptions and nuances:
-
d-block contraction: The filling of the d-orbitals results in a less effective shielding effect, leading to a slightly smaller atomic radius than expected based on simple trends. This is because d electrons are not as effective at shielding outer electrons from the nuclear charge.
-
Lanthanide contraction: This is a similar effect to d-block contraction, but more pronounced. The poor shielding effect of the f-orbitals leads to a significant reduction in atomic radius across the lanthanide series. This contraction affects the sizes of the elements following the lanthanides in the periodic table.
-
Relativistic effects: At very high atomic numbers, relativistic effects become significant. These effects alter the electron velocities and distributions, influencing atomic size. This is most prominent for heavy elements.
Practical Applications of Understanding Atomic Radius
Understanding atomic radius is crucial in several areas:
-
Chemical Bonding: The size of atoms greatly influences bond lengths, bond strengths, and the overall geometry of molecules. Larger atoms form longer bonds and often have weaker bonds.
-
Material Science: Atomic radius is a factor in determining the properties of materials. For instance, it influences the density, melting point, and electrical conductivity of materials.
-
Catalysis: The size and shape of catalytic sites often depend on the atomic radii of the constituent atoms.
-
Nuclear Physics: Atomic radii are relevant in understanding nuclear reactions and interactions.
Conclusion
Determining which atom has the largest atomic radius depends heavily on its position in the periodic table. While general trends indicate that elements lower in a group and further left in a period possess larger atomic radii, various factors, such as nuclear charge, electron shielding, and relativistic effects, can subtly influence the size. A comprehensive understanding of these factors is crucial for accurately predicting relative atomic sizes and for appreciating their implications across various scientific disciplines. Remember to always consider the interplay of these factors when comparing atomic radii and avoid oversimplifying the determination of atomic size. Further exploration of these topics will solidify your understanding of this fundamental concept in chemistry.
Latest Posts
Related Post
Thank you for visiting our website which covers about Of The Following Which Atom Has The Largest Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.