What Is The Conjugate Base Of H2po4
News Leon
Mar 26, 2025 · 6 min read
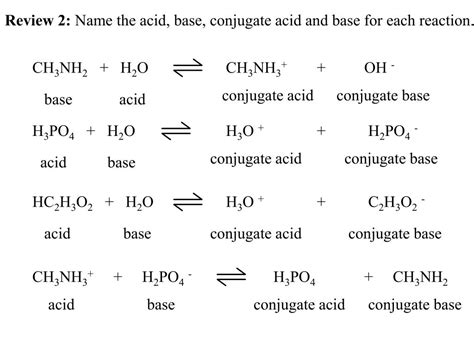
Table of Contents
What is the Conjugate Base of H₂PO₄⁻? Understanding Acid-Base Chemistry
The question, "What is the conjugate base of H₂PO₄⁻?" delves into the fundamental concepts of acid-base chemistry, specifically the Brønsted-Lowry theory. Understanding conjugate acid-base pairs is crucial for predicting reaction outcomes and manipulating chemical equilibria. This article will thoroughly explore the conjugate base of dihydrogen phosphate (H₂PO₄⁻), its properties, and its significance in various chemical systems. We'll also touch upon related concepts like polyprotic acids and their conjugate bases to provide a comprehensive understanding of this topic.
Understanding Brønsted-Lowry Theory
Before diving into the specifics of H₂PO₄⁻, let's refresh our understanding of the Brønsted-Lowry theory. This theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. Crucially, the theory introduces the concept of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base, which is the species remaining after the proton is lost. Conversely, when a base accepts a proton, it forms its conjugate acid. The conjugate acid-base pair differs by only a single proton (H⁺).
Identifying the Conjugate Base of H₂PO₄⁻
Dihydrogen phosphate (H₂PO₄⁻) acts as a weak acid in aqueous solutions. This means it only partially donates its protons. When H₂PO₄⁻ donates a proton (H⁺), it forms its conjugate base. The reaction can be represented as follows:
H₂PO₄⁻ (aq) ⇌ H⁺ (aq) + HPO₄²⁻ (aq)
In this equilibrium, H₂PO₄⁻ acts as the acid, donating a proton to form its conjugate base, HPO₄²⁻ (hydrogen phosphate). The hydrogen phosphate ion (HPO₄²⁻) is one proton less than the dihydrogen phosphate ion (H₂PO₄⁻), fulfilling the definition of a conjugate base.
Key Characteristics of HPO₄²⁻ (Hydrogen Phosphate)
- Charge: HPO₄²⁻ carries a -2 charge, one more negative than its conjugate acid H₂PO₄⁻. This increased negative charge reflects the acceptance of a proton by the conjugate base.
- Reactivity: HPO₄²⁻ can act as both a base (accepting a proton) and an acid (donating a proton), demonstrating its amphoteric nature. It can accept a proton to form H₂PO₄⁻ or donate a proton to form PO₄³⁻ (phosphate).
- Presence in Biological Systems: HPO₄²⁻ plays a crucial role in biological systems. It's a major component of buffers in bodily fluids, maintaining a stable pH for optimal biological function. This is due to its ability to act as both an acid and a base, resisting changes in pH.
Phosphoric Acid and its Conjugate Bases: A Polyprotic Perspective
Understanding the conjugate base of H₂PO₄⁻ requires considering its parent acid, phosphoric acid (H₃PO₄). Phosphoric acid is a triprotic acid, meaning it can donate three protons sequentially. Each proton donation results in a new conjugate base. Let's examine the stepwise deprotonation:
1. First Deprotonation:
H₃PO₄ (aq) ⇌ H⁺ (aq) + H₂PO₄⁻ (aq)
Here, phosphoric acid (H₃PO₄) donates one proton to form its conjugate base, dihydrogen phosphate (H₂PO₄⁻).
2. Second Deprotonation:
H₂PO₄⁻ (aq) ⇌ H⁺ (aq) + HPO₄²⁻ (aq)
Dihydrogen phosphate (H₂PO₄⁻) acts as an acid, donating a proton to form its conjugate base, hydrogen phosphate (HPO₄²⁻). This is the specific conjugate base we've been focusing on.
3. Third Deprotonation:
HPO₄²⁻ (aq) ⇌ H⁺ (aq) + PO₄³⁻ (aq)
Hydrogen phosphate (HPO₄²⁻) acts as an acid, donating its last proton to form its conjugate base, phosphate (PO₄³⁻).
This stepwise deprotonation demonstrates that the conjugate base of one species can act as an acid itself, leading to further deprotonation. This is a common characteristic of polyprotic acids.
The Importance of Conjugate Bases in Buffer Solutions
Conjugate acid-base pairs play a crucial role in creating buffer solutions. Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. The effectiveness of a buffer solution depends on the concentration of the conjugate acid-base pair and its pKa value (acid dissociation constant).
The H₂PO₄⁻/HPO₄²⁻ conjugate pair is a particularly important buffer system in biological systems. It helps to maintain the pH of intracellular and extracellular fluids within a narrow range, essential for the proper functioning of enzymes and other biomolecules. The ability of HPO₄²⁻ to act as both an acid and a base makes it highly effective in buffering against both acid and base additions.
Applications of HPO₄²⁻ and its Conjugate Acid
The H₂PO₄⁻/HPO₄²⁻ conjugate pair finds applications in various fields, beyond its crucial biological role:
- Agriculture: Phosphates, including HPO₄²⁻, are essential nutrients for plant growth. They are commonly used in fertilizers to increase crop yields.
- Food Industry: Phosphates are used as food additives to enhance the texture, taste, and shelf life of food products.
- Water Treatment: Phosphates are used in water treatment processes to control hardness and prevent scale formation in pipes and equipment.
- Chemical Industry: HPO₄²⁻ and related compounds are used in the synthesis of various chemicals, including detergents and cleaning agents.
Acid Dissociation Constant (Ka) and pKa: Quantifying Acid Strength
The strength of an acid, and consequently the relative stability of its conjugate base, is quantified using the acid dissociation constant (Ka). The Ka value represents the equilibrium constant for the dissociation of an acid in water. A higher Ka value indicates a stronger acid, implying a weaker conjugate base. The pKa, which is the negative logarithm of Ka, is often used as a more convenient measure of acid strength. A lower pKa value indicates a stronger acid and a weaker conjugate base.
The pKa values for the stepwise dissociation of phosphoric acid are:
- H₃PO₄: pKa1 ≈ 2.15
- H₂PO₄⁻: pKa2 ≈ 7.20
- HPO₄²⁻: pKa3 ≈ 12.35
These values demonstrate that H₂PO₄⁻ is a weaker acid than H₃PO₄, and HPO₄²⁻ is even weaker. This progression reflects the increasing difficulty in removing a proton from increasingly negatively charged species.
Conclusion: A Deeper Understanding of Conjugate Bases
In conclusion, the conjugate base of H₂PO₄⁻ is HPO₄²⁻ (hydrogen phosphate). Understanding this relationship is fundamental to grasping acid-base chemistry, particularly within the context of the Brønsted-Lowry theory. The amphoteric nature of HPO₄²⁻, its role in buffering solutions, and its widespread applications in various fields highlight its significance. By exploring the stepwise deprotonation of phosphoric acid, we gain a broader perspective on polyprotic acids and their conjugate bases. This knowledge is crucial for anyone studying chemistry, biochemistry, or related fields. The detailed analysis of Ka and pKa values helps quantify the strength of acids and the relative stability of their conjugate bases, allowing for a more precise understanding of chemical equilibria and reaction outcomes. This comprehensive overview provides a solid foundation for further exploration of acid-base chemistry and its practical applications.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Is The Conjugate Base Of H2po4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.