Which Of The Following Mixtures Are Solutions
News Leon
Mar 16, 2025 · 6 min read
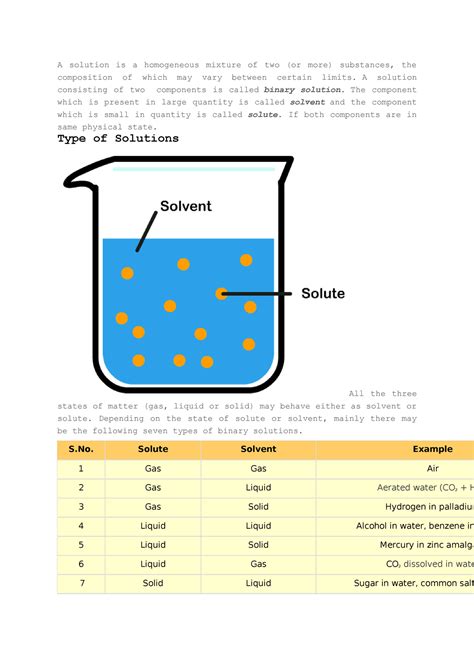
Table of Contents
Which of the Following Mixtures are Solutions? A Deep Dive into Solution Chemistry
Understanding the difference between mixtures and solutions is fundamental to chemistry. While all solutions are mixtures, not all mixtures are solutions. This crucial distinction lies in the size and distribution of the particles within the mixture. This article will explore the characteristics of solutions, differentiating them from other types of mixtures like suspensions and colloids, and provide examples to clarify the concepts. We'll also delve into the factors affecting solubility and the various types of solutions.
What is a Solution?
A solution is a homogeneous mixture composed of two or more substances. The key characteristic of a solution is its uniformity at the molecular level. This means that the components of the solution are completely and evenly dispersed, creating a single phase. You won't be able to visually distinguish the different components. The substance present in the larger amount is called the solvent, and the substance dissolved in the solvent is the solute. For example, in saltwater, water is the solvent and salt is the solute.
Key Properties of Solutions:
- Homogeneity: Solutions are uniform throughout. No matter where you sample from the solution, the composition will be identical.
- Particle Size: The solute particles are extremely small, typically at the atomic or molecular level (less than 1 nanometer). This small size contributes to their inability to be filtered or separated by simple methods like decantation or sedimentation.
- Transparency: True solutions are usually transparent, allowing light to pass through without scattering. You can see through a solution.
- Stability: Solutions are generally stable, meaning the solute doesn't settle out over time. The solute remains dissolved.
- Filtration: Solutions cannot be separated by simple filtration because the solute particles are too small to be trapped by filter paper.
Differentiating Solutions from Other Mixtures: Suspensions and Colloids
To fully understand solutions, it's essential to compare them with other types of mixtures: suspensions and colloids. These mixtures differ primarily in the size of their dispersed particles.
Suspensions:
Suspensions are heterogeneous mixtures where the solute particles are much larger than those in solutions. These particles are easily visible to the naked eye and will settle out of the solution over time due to gravity. They can also be separated by simple filtration. Examples include muddy water, sand in water, and paint.
Key Characteristics of Suspensions:
- Heterogeneity: Not uniform throughout; different regions have different compositions.
- Large Particle Size: Particles are larger than 1000 nanometers.
- Settling: Particles settle out upon standing.
- Filtration: Particles can be separated by filtration.
- Opacity: Suspensions are often opaque; light cannot pass through.
Colloids:
Colloids fall between solutions and suspensions in terms of particle size. The dispersed particles are larger than in solutions but smaller than in suspensions (between 1 and 1000 nanometers). They don't settle out easily and cannot be easily separated by filtration. Examples include milk, fog, and blood.
Key Characteristics of Colloids:
- Heterogeneity: While appearing homogeneous at first glance, colloids are actually heterogeneous at the microscopic level.
- Intermediate Particle Size: Particles are between 1 and 1000 nanometers.
- Tyndall Effect: Colloids exhibit the Tyndall effect, scattering light passing through them. This creates a visible beam of light (like a sunbeam through fog).
- Stability: Colloids are relatively stable; particles don't settle quickly.
- Filtration: Particles are too small to be easily separated by simple filtration.
Factors Affecting Solubility: The "Like Dissolves Like" Rule
The ability of a substance to dissolve in a solvent is called solubility. The "like dissolves like" rule is a useful guideline. Polar solvents tend to dissolve polar solutes, and nonpolar solvents tend to dissolve nonpolar solutes.
- Polarity: Polar molecules have a positive and negative end due to differences in electronegativity between atoms. Water is a classic example of a polar solvent.
- Nonpolarity: Nonpolar molecules have an even distribution of charge. Examples include oils and fats.
- Temperature: Increasing temperature generally increases the solubility of solids in liquids. However, the effect of temperature on gas solubility is the opposite; increasing temperature decreases the solubility of gases in liquids.
- Pressure: Pressure significantly affects the solubility of gases in liquids. Increasing pressure increases gas solubility (Henry's Law).
Types of Solutions:
Solutions can be categorized based on the physical state of the solute and solvent.
- Gaseous Solutions: Both the solute and solvent are gases (e.g., air—a mixture of nitrogen, oxygen, and other gases).
- Liquid Solutions: The solvent is a liquid. This is the most common type of solution (e.g., saltwater, sugar in water, alcohol in water).
- Solid Solutions: The solvent is a solid (e.g., alloys like brass—a mixture of copper and zinc).
Examples to Illustrate the Concepts:
Let's analyze some mixtures to determine if they are solutions:
1. Saltwater: This is a solution. Salt (NaCl) dissolves completely in water, forming a homogeneous mixture at the molecular level. The salt ions are evenly dispersed throughout the water.
2. Sand in Water: This is a suspension. Sand particles are much larger than water molecules and will settle at the bottom upon standing. They can be easily separated by filtration.
3. Milk: This is a colloid. Milk contains fat globules and proteins dispersed in water. These particles are larger than molecules but smaller than those in a suspension. Milk exhibits the Tyndall effect.
4. Sugar in Water: This is a solution. Sugar dissolves completely in water, creating a homogeneous mixture.
5. Air: This is a gaseous solution. Various gases like oxygen, nitrogen, and carbon dioxide are uniformly mixed.
6. Brass: This is a solid solution (alloy). Copper and zinc atoms are uniformly mixed in the solid state.
7. Muddy Water: This is a suspension. Mud particles are visible and will settle over time.
8. Fog: This is a colloid. Tiny water droplets are suspended in air, exhibiting the Tyndall effect.
9. Vinegar: This is a solution. Acetic acid (the solute) is dissolved in water (the solvent).
10. Blood: This is a colloid. Blood contains various cells and proteins suspended in plasma.
Conclusion:
Understanding the distinction between solutions, suspensions, and colloids is crucial in chemistry and many other scientific fields. Solutions, with their uniform composition at the molecular level and small particle size, are distinct from other mixtures. By understanding the factors affecting solubility and the different types of solutions, we can better predict the behavior of mixtures and apply this knowledge to various applications. Remember the key characteristics – homogeneity, particle size, transparency, stability, and filtration behavior – to identify whether a given mixture is a solution. This knowledge helps in various applications, from designing medications to understanding environmental processes.
Latest Posts
Latest Posts
-
How To Calculate E Not Cell
Mar 17, 2025
-
What Part Of The Ear Looks Like A Snail Shell
Mar 17, 2025
-
What Is The Molar Mass Of Agno3
Mar 17, 2025
-
What Percent Of 68 Is 17
Mar 17, 2025
-
Which Bond Is The Most Polar
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Mixtures Are Solutions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
