How To Calculate E Not Cell
News Leon
Mar 17, 2025 · 6 min read
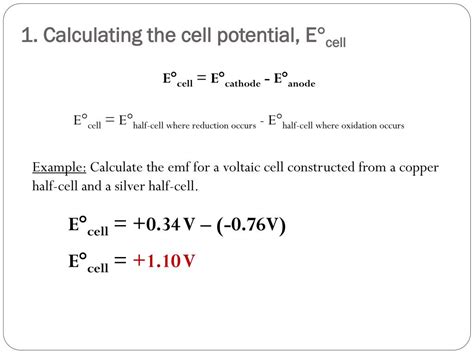
Table of Contents
How to Calculate E°<sub>cell</sub>: A Comprehensive Guide
Calculating the standard cell potential, E°<sub>cell</sub>, is a fundamental concept in electrochemistry. It allows us to predict the spontaneity of a redox reaction and the voltage generated by a galvanic cell. This comprehensive guide will walk you through the process, explaining the underlying principles and offering various examples. We'll explore different methods, focusing on clarity and practical application.
Understanding E°<sub>cell</sub>
The standard cell potential (E°<sub>cell</sub>) represents the potential difference between the two half-cells of an electrochemical cell under standard conditions: 298 K (25°C), 1 atm pressure, and 1 M concentration for all aqueous solutions. A positive E°<sub>cell</sub> indicates a spontaneous reaction (galvanic cell), while a negative E°<sub>cell</sub> suggests a non-spontaneous reaction (electrolytic cell) requiring external energy to proceed.
Calculating E°<sub>cell</sub> Using Standard Reduction Potentials
The most common method for calculating E°<sub>cell</sub> involves using standard reduction potentials (E°<sub>red</sub>). These values are tabulated for various half-reactions, representing the tendency of a species to gain electrons under standard conditions. Remember, reduction always occurs at the cathode and oxidation at the anode.
The Key Formula:
The core equation for calculating E°<sub>cell</sub> is:
E°<sub>cell</sub> = E°<sub>cathode</sub> - E°<sub>anode</sub>
Where:
- E°<sub>cathode</sub> is the standard reduction potential of the reduction half-reaction (cathode).
- E°<sub>anode</sub> is the standard reduction potential of the oxidation half-reaction (anode). Important: Since we're dealing with the oxidation half-reaction at the anode, you must reverse the sign of the standard reduction potential listed in tables.
Step-by-Step Procedure:
-
Identify the Half-Reactions: Write the balanced half-reactions for both oxidation and reduction occurring in the electrochemical cell.
-
Find Standard Reduction Potentials: Consult a standard reduction potential table (available in most chemistry textbooks and online resources) to find the E°<sub>red</sub> values for each half-reaction.
-
Reverse the Oxidation Half-Reaction's Potential: Change the sign of the E°<sub>red</sub> value for the half-reaction occurring at the anode (oxidation).
-
Apply the Formula: Substitute the E°<sub>red</sub> values (with the sign change for the anode) into the E°<sub>cell</sub> formula and calculate.
Example 1: Zn-Cu Cell
Consider a galvanic cell with a zinc anode (Zn) and a copper cathode (Cu). The half-reactions are:
- Anode (Oxidation): Zn(s) → Zn<sup>2+</sup>(aq) + 2e<sup>-</sup> E°<sub>ox</sub> = +0.76 V (Reversed sign from standard reduction potential)
- Cathode (Reduction): Cu<sup>2+</sup>(aq) + 2e<sup>-</sup> → Cu(s) E°<sub>red</sub> = +0.34 V
Applying the formula:
E°<sub>cell</sub> = E°<sub>cathode</sub> - E°<sub>anode</sub> = (+0.34 V) - (-0.76 V) = +1.10 V
The positive E°<sub>cell</sub> confirms the spontaneity of the reaction under standard conditions.
Example 2: Fe-Ag Cell
Let's analyze a cell with an iron (Fe) anode and a silver (Ag) cathode.
- Anode (Oxidation): Fe(s) → Fe<sup>2+</sup>(aq) + 2e<sup>-</sup> E°<sub>ox</sub> = +0.44 V (Reversed sign)
- Cathode (Reduction): Ag<sup>+</sup>(aq) + e<sup>-</sup> → Ag(s) E°<sub>red</sub> = +0.80 V
However, notice that the number of electrons transferred is different. Before applying the formula, we need to balance the electrons:
Multiply the silver half-reaction by 2: 2Ag<sup>+</sup>(aq) + 2e<sup>-</sup> → 2Ag(s)
Now we can calculate:
E°<sub>cell</sub> = E°<sub>cathode</sub> - E°<sub>anode</sub> = (+0.80 V) - (+0.44V) = +0.36 V
Again, the positive value confirms spontaneity.
Dealing with Non-Standard Conditions: The Nernst Equation
The E°<sub>cell</sub> values are calculated under standard conditions. However, real-world applications rarely operate under these ideal circumstances. To account for variations in temperature and concentration, we use the Nernst equation:
E<sub>cell</sub> = E°<sub>cell</sub> - (RT/nF)lnQ
Where:
- E<sub>cell</sub> is the cell potential under non-standard conditions.
- E°<sub>cell</sub> is the standard cell potential.
- R is the ideal gas constant (8.314 J/mol·K).
- T is the temperature in Kelvin.
- n is the number of moles of electrons transferred in the balanced redox reaction.
- F is the Faraday constant (96485 C/mol).
- Q is the reaction quotient.
The reaction quotient (Q) is the ratio of the concentrations of products to reactants, raised to the powers of their stoichiometric coefficients, similar to the equilibrium constant (K) but at any point during the reaction.
Example 3: Applying the Nernst Equation
Let's reconsider the Zn-Cu cell but with non-standard concentrations:
[Zn<sup>2+</sup>] = 0.1 M and [Cu<sup>2+</sup>] = 1.0 M at 298 K.
From Example 1, we know E°<sub>cell</sub> = +1.10 V. The balanced reaction is:
Zn(s) + Cu<sup>2+</sup>(aq) → Zn<sup>2+</sup>(aq) + Cu(s)
n = 2 (two electrons transferred)
Q = [Zn<sup>2+</sup>]/[Cu<sup>2+</sup>] = 0.1 M / 1.0 M = 0.1
Substituting into the Nernst equation:
E<sub>cell</sub> = 1.10 V - [(8.314 J/mol·K)(298 K) / (2 mol)(96485 C/mol)] ln(0.1)
After calculation, you will find E<sub>cell</sub> > E°<sub>cell</sub>, reflecting the influence of the concentration difference. A higher concentration of Cu<sup>2+</sup> compared to Zn<sup>2+</sup> drives the reaction further towards products, increasing the cell potential.
Troubleshooting Common Calculation Mistakes
- Incorrect Sign for Anode Potential: Remember to reverse the sign of the standard reduction potential for the oxidation half-reaction (anode). This is a very common error.
- Electron Balancing: Always ensure the number of electrons lost in oxidation equals the number gained in reduction before calculating E°<sub>cell</sub>. If the electron numbers differ, you must balance the half-reactions before proceeding.
- Units: Be consistent with units in the Nernst equation. Use Kelvin for temperature and ensure all concentration terms are in molarity (M).
- Reaction Quotient (Q): Carefully construct the reaction quotient (Q), ensuring correct stoichiometric coefficients and concentrations of reactants and products.
- Significant Figures: Pay attention to significant figures throughout your calculations, ensuring your final answer reflects the precision of your input values.
Advanced Considerations
- Activity vs. Concentration: At higher concentrations, using activity instead of concentration in the Nernst equation provides a more accurate calculation. Activity accounts for intermolecular interactions that affect the effective concentration of ions.
- Temperature Dependence: The Nernst equation assumes a constant temperature. For significant temperature variations, more complex thermodynamic equations might be necessary.
- Non-Ideal Behavior: Deviations from ideality can occur at high concentrations or in solutions with complex interactions. Corrections might be required for highly accurate calculations.
- Different types of electrodes: The calculation of E°<sub>cell</sub> can be modified slightly depending on the nature of the electrodes used.
Conclusion
Calculating E°<sub>cell</sub> is a crucial skill in electrochemistry, enabling predictions of reaction spontaneity and cell potential. Mastering the techniques outlined in this guide, from utilizing standard reduction potentials to applying the Nernst equation for non-standard conditions, provides a strong foundation for understanding electrochemical processes. Remember to pay close attention to details, especially regarding signs and balancing electrons, and always double-check your work to avoid common errors. This meticulous approach will ensure accurate and reliable results. By consistently applying these principles, you'll confidently navigate the intricacies of electrochemical calculations.
Latest Posts
Latest Posts
-
Did The Ussr Imiss The Great Depression
Mar 17, 2025
-
Choose The Components Of A Respiratory Membrane
Mar 17, 2025
-
Dendrite Is To Axon As Is To
Mar 17, 2025
-
The Quotient Of A Number And 2
Mar 17, 2025
-
Which Of The Following Is The Most Stable Carbocation
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate E Not Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
