Which Bond Is The Most Polar
News Leon
Mar 17, 2025 · 5 min read
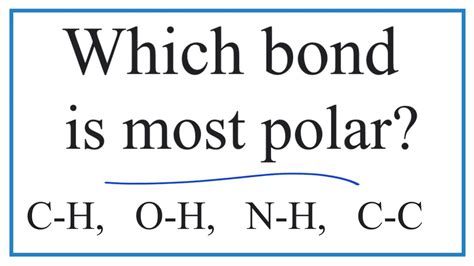
Table of Contents
Which Bond is the Most Polar? Understanding Electronegativity and Bond Polarity
The question of which bond is the most polar is a fascinating one that delves into the fundamental principles of chemistry. It's not a simple matter of picking one bond and declaring it the "most" polar, as polarity is a spectrum, not a discrete category. However, we can explore the concept of electronegativity, the driving force behind bond polarity, and examine some of the most polar bonds found in nature. Understanding this concept is crucial for comprehending many aspects of chemistry, from molecular geometry to reactivity.
Understanding Electronegativity
Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with high electronegativity strongly pull shared electrons closer to their nucleus, creating a dipole moment. This dipole moment is the essence of bond polarity. The greater the difference in electronegativity between two bonded atoms, the more polar the bond will be.
Several electronegativity scales exist, the most commonly used being the Pauling scale. On this scale, fluorine (F) holds the highest electronegativity value (approximately 4.0), indicating its exceptional ability to attract electrons. Other highly electronegative elements include oxygen (O), nitrogen (N), and chlorine (Cl). Elements with low electronegativity, such as alkali metals and alkaline earth metals, readily lose electrons.
The Pauling Electronegativity Scale and its Limitations
While the Pauling scale is widely used and readily accessible, it's crucial to understand its limitations. It's an empirical scale, meaning it's based on observed properties rather than a purely theoretical calculation. Also, the values are relative; they represent the electronegativity of an element compared to other elements. The absolute electronegativity of an element remains difficult to define precisely. Different scales, such as the Mulliken scale and the Allred-Rochow scale, exist, offering slightly different values, but the overall trends remain consistent.
Factors Influencing Bond Polarity
Besides the difference in electronegativity, other factors subtly influence bond polarity:
-
Bond Length: Shorter bonds generally exhibit greater polarity because the electrons are held more closely to the more electronegative atom.
-
Hybridization: The hybridization of the atomic orbitals involved in bond formation can affect the electron distribution and, consequently, the bond polarity. For instance, sp hybridized orbitals are more electronegative than sp<sup>3</sup> hybridized orbitals.
-
Molecular Environment: The surrounding atoms and molecules in a molecule can influence the electron distribution, slightly altering bond polarity. This effect is often minor compared to the electronegativity difference.
Identifying the Most Polar Bonds: A Case Study
While a definitive "most polar" bond is debatable due to the subtle variations and limitations of electronegativity scales, we can analyze some strong contenders. Bonds involving fluorine often top the list due to fluorine's exceptionally high electronegativity.
Hydrogen Fluoride (HF): A Prime Example
The bond in hydrogen fluoride (HF) is often cited as one of the most polar bonds. The electronegativity difference between fluorine (4.0) and hydrogen (2.1) is significant (approximately 1.9), resulting in a substantial dipole moment. The highly electronegative fluorine atom strongly pulls the shared electrons towards itself, leaving the hydrogen atom with a partial positive charge (δ+) and the fluorine atom with a partial negative charge (δ−). This significant polarity contributes to HF's high boiling point and its ability to act as a strong hydrogen bond donor.
Other Strong Contenders
Other bonds that exhibit high polarity include:
-
Hydrogen-Oxygen (O-H): Found in water (H₂O) and alcohols, this bond displays a substantial electronegativity difference, leading to significant polarity. The polarity of O-H bonds is responsible for the unique properties of water, such as its high boiling point and its ability to act as both a hydrogen bond donor and acceptor.
-
Carbon-Fluorine (C-F): The C-F bond is exceptionally polar due to the large electronegativity difference between carbon and fluorine. This makes fluorocarbons useful in various applications, including refrigerants and pharmaceuticals. The high polarity of the C-F bond contributes to the unique properties of fluorocarbons, such as their low reactivity and high stability.
-
Hydrogen-Nitrogen (N-H): This bond is present in ammonia (NH₃) and amines. The electronegativity difference, although smaller than in HF or O-H, still leads to noticeable polarity. This polarity contributes to the ability of ammonia to act as a base and form hydrogen bonds.
-
Hydrogen-Chlorine (H-Cl): Hydrogen chloride (HCl) exhibits a considerable electronegativity difference, creating a polar bond. This polarity contributes to HCl's solubility in water and its acidic properties.
Important Note: The degree of polarity is a continuous spectrum. The examples above represent some of the most polar bonds, but many other bonds possess significant polarity.
The Significance of Polar Bonds in Chemistry
The polarity of a bond significantly impacts a molecule's overall properties and behavior. Understanding bond polarity is essential for predicting:
-
Molecular Geometry: Polar bonds can influence the overall molecular shape due to dipole-dipole interactions.
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents.
-
Boiling and Melting Points: Polar molecules generally have higher boiling and melting points than nonpolar molecules due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding).
-
Reactivity: The polarity of a bond determines its susceptibility to attack by nucleophiles or electrophiles.
-
Spectroscopic Properties: Polar bonds exhibit characteristic absorption patterns in infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy, which are crucial techniques for identifying and characterizing molecules.
Conclusion: A Spectrum of Polarity
While pinpointing the single "most" polar bond is challenging due to the nuanced nature of electronegativity and other influencing factors, bonds involving fluorine, particularly the H-F bond, are consistently recognized as among the most polar. Understanding the concept of electronegativity and its effect on bond polarity is crucial for comprehending a wide array of chemical phenomena. The polarity of a bond is not a binary property but exists on a spectrum, significantly impacting the physical and chemical properties of molecules. By understanding this spectrum, chemists can better predict and explain the behavior of various compounds and materials. Further research into more precise electronegativity scales and computational modelling could provide a more refined understanding of bond polarity, but the fundamental principles remain clear: the larger the difference in electronegativity between two atoms, the more polar the bond they form.
Latest Posts
Latest Posts
-
What Kind Of Acid Is In A Car Battery
Mar 17, 2025
-
What Is The Molar Mass Of Ca Oh 2
Mar 17, 2025
-
Which Of These Structures Is Diploid
Mar 17, 2025
-
How Many Light Years Is Earth From Mars
Mar 17, 2025
-
Serous Membrane That Covers The Lungs
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which Bond Is The Most Polar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
