What Kind Of Acid Is In A Car Battery
News Leon
Mar 17, 2025 · 5 min read
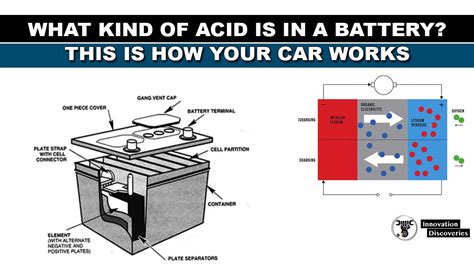
Table of Contents
What Kind of Acid is in a Car Battery? Understanding Sulfuric Acid and its Role
Car batteries are essential components of modern vehicles, providing the electrical power needed to start the engine and run various accessories. But what makes these batteries work? The answer lies in a potent and corrosive substance: sulfuric acid. This article delves deep into the nature of sulfuric acid in car batteries, exploring its properties, its role in the battery's operation, safety precautions, and environmental considerations.
Understanding Sulfuric Acid: The Workhorse of Car Batteries
Sulfuric acid (H₂SO₄) is a strong mineral acid, meaning it readily donates protons (H⁺ ions) in aqueous solutions. It's a highly corrosive substance, capable of causing severe burns upon contact with skin or eyes. Its strength lies in its ability to completely dissociate into ions in water, forming hydronium ions (H₃O⁺) and sulfate ions (SO₄²⁻). This high degree of ionization is key to its functionality in car batteries.
Key Properties of Sulfuric Acid Relevant to Battery Function:
- High Ion Concentration: The complete dissociation of sulfuric acid in water creates a high concentration of ions, which are crucial for conducting electricity within the battery. The flow of these ions is what facilitates the electrical current.
- Electrolyte Role: Sulfuric acid acts as the electrolyte in lead-acid batteries, allowing the flow of ions between the electrodes (lead plates) during charging and discharging. Without this electrolyte, no current would flow.
- Oxidizing and Reducing Properties: Sulfuric acid participates in the redox (reduction-oxidation) reactions that occur within the battery. It gets reduced at the cathode and oxidized at the anode during discharge. This interplay of oxidation and reduction is the fundamental principle behind the battery's operation.
- High Boiling Point: The relatively high boiling point of sulfuric acid ensures it remains liquid within the battery over a wide range of temperatures, ensuring consistent performance across various climates.
The Lead-Acid Battery: A Deep Dive into its Chemistry
Car batteries are predominantly lead-acid batteries, employing a specific chemical reaction involving lead, lead(IV) oxide, and sulfuric acid. Understanding this reaction is essential to grasping the role of sulfuric acid.
The Battery's Components:
- Lead Plates (Anode and Cathode): The battery consists of several lead plates acting as electrodes. The anode is made of pure lead (Pb), while the cathode is composed of lead(IV) oxide (PbO₂).
- Sulfuric Acid Solution (Electrolyte): The plates are immersed in an aqueous solution of sulfuric acid, which serves as the electrolyte.
- Separator: A porous separator prevents direct contact between the anode and cathode, while allowing ion transport.
The Discharge Reaction:
During discharge (when the battery powers the car), the following reaction occurs:
Pb(s) + PbO₂(s) + 2H₂SO₄(aq) ⇌ 2PbSO₄(s) + 2H₂O(l)
- Anode (Oxidation): Lead (Pb) at the anode loses electrons, oxidizing to lead(II) sulfate (PbSO₄).
- Cathode (Reduction): Lead(IV) oxide (PbO₂) at the cathode gains electrons, reducing to lead(II) sulfate (PbSO₄).
- Sulfuric Acid Consumption: Sulfuric acid is consumed during the discharge process, forming water as a byproduct. This leads to a decrease in the concentration of sulfuric acid and a corresponding decrease in the battery's voltage.
The Charging Reaction:
When the battery is connected to a charger, the process reverses:
2PbSO₄(s) + 2H₂O(l) ⇌ Pb(s) + PbO₂(s) + 2H₂SO₄(aq)
- Anode (Reduction): Lead(II) sulfate (PbSO₄) at the anode gains electrons, reducing to lead (Pb).
- Cathode (Oxidation): Lead(II) sulfate (PbSO₄) at the cathode loses electrons, oxidizing to lead(IV) oxide (PbO₂).
- Sulfuric Acid Regeneration: Sulfuric acid is regenerated during the charging process, increasing its concentration and restoring the battery's voltage.
Safety Precautions When Handling Car Batteries and Sulfuric Acid
Sulfuric acid is a hazardous substance; therefore, extreme caution is necessary when handling car batteries.
Potential Hazards:
- Corrosive Nature: Direct contact with sulfuric acid can cause severe burns to skin, eyes, and other tissues. Inhalation of sulfuric acid mist can also be harmful to the respiratory system.
- Exothermic Reaction with Water: Mixing sulfuric acid with water is highly exothermic (heat-releasing). Always add acid to water slowly and carefully, never the other way around, to avoid splashing and potential burns.
- Hydrogen Gas Generation: During charging, hydrogen gas (H₂) can be produced. Hydrogen is highly flammable and can form explosive mixtures with air. Ensure proper ventilation when charging batteries.
Safety Measures:
- Protective Gear: Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat or apron when handling car batteries or sulfuric acid.
- Ventilation: Work in a well-ventilated area to prevent the buildup of hydrogen gas.
- Proper Disposal: Dispose of used car batteries and spent sulfuric acid according to local regulations. Never pour acid down the drain or into the environment.
- Spills: In case of a spill, neutralize the acid with a base like sodium bicarbonate (baking soda) and clean up the area thoroughly. Consult relevant safety data sheets for specific spill procedures.
Environmental Considerations: Recycling and Responsible Disposal
The improper disposal of car batteries poses a significant environmental risk due to the presence of lead and sulfuric acid.
Environmental Impact:
- Lead Toxicity: Lead is a heavy metal that is highly toxic to humans and wildlife. Lead contamination of soil and water can have severe consequences for ecosystems.
- Acidification: Sulfuric acid is an acidic substance that can contribute to soil and water acidification, harming plant and animal life.
Responsible Disposal and Recycling:
- Recycling Programs: Many communities have battery recycling programs that collect used car batteries for safe disposal and recycling. Participate in these programs to minimize environmental impact.
- Proper Handling: Always handle car batteries and spent acid carefully to prevent spills and contamination.
- Manufacturer Responsibility: Many battery manufacturers have taken responsibility for the recycling of their products, implementing comprehensive take-back programs.
Conclusion: The Crucial Role of Sulfuric Acid in Modern Transportation
Sulfuric acid is the lifeblood of the lead-acid battery, enabling its ability to store and release electrical energy. Understanding its properties, the chemical reactions involved, and the necessary safety precautions is crucial for responsible handling and environmental stewardship. While the acid itself presents potential hazards, responsible recycling programs and conscientious practices help mitigate its environmental impact, allowing for the continued use of this essential component in modern transportation. Remember, proper handling and disposal are not just safety concerns, but crucial steps towards protecting our environment. The future of transportation relies on a balance between technological advancements and responsible environmental stewardship.
Latest Posts
Latest Posts
-
0 3 To The Power Of 3
Mar 17, 2025
-
What Is 5 Percent Of 200
Mar 17, 2025
-
What Is The 5 Difference Between Photosynthesis And Respiration
Mar 17, 2025
-
Which Of The Following Is A Characteristic Of Cnidarians
Mar 17, 2025
-
Metallic Trends In The Periodic Table
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Kind Of Acid Is In A Car Battery . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
