Metallic Trends In The Periodic Table
News Leon
Mar 17, 2025 · 5 min read
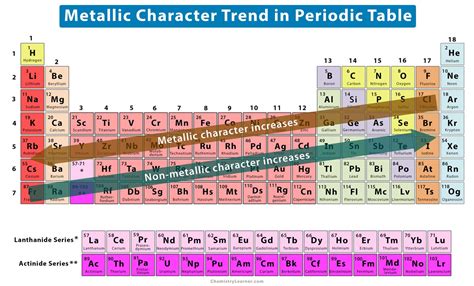
Table of Contents
Metallic Trends in the Periodic Table: A Comprehensive Exploration
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. Understanding these properties, particularly metallic character, is crucial for comprehending chemical reactions, material science, and the behavior of various substances. This article delves into the fascinating trends of metallic character across the periodic table, exploring its origins, variations, and consequences.
What is Metallic Character?
Metallic character refers to the degree to which an element exhibits properties characteristic of metals. These properties include:
- Electrical conductivity: Metals are excellent conductors of electricity due to the ease with which their valence electrons can move freely.
- Thermal conductivity: Metals efficiently transfer heat due to the mobile nature of their electrons.
- Malleability: Metals can be hammered into thin sheets without breaking.
- Ductility: Metals can be drawn into wires.
- Luster: Metals possess a characteristic shine.
- Low ionization energy: Metals readily lose electrons to form positive ions (cations).
- Low electronegativity: Metals have a low tendency to attract electrons.
Periodic Trends in Metallic Character
Metallic character follows specific trends across the periodic table, primarily influenced by two factors: atomic radius and effective nuclear charge.
1. Atomic Radius: The Size Matters
Atomic radius increases as you move down a group and decreases as you move across a period (from left to right). This trend stems from the addition of electron shells (down a group) and the increasing nuclear charge attracting electrons more strongly (across a period).
-
Down a group: Larger atomic radii lead to increased metallic character. The outermost electrons are farther from the nucleus, experiencing weaker attraction, making them easier to lose and thus exhibiting more metallic properties.
-
Across a period: Decreasing atomic radii leads to decreased metallic character. The stronger nuclear attraction holds the valence electrons more tightly, making them less likely to be lost, resulting in less metallic behavior.
2. Effective Nuclear Charge: The Pull of the Nucleus
Effective nuclear charge refers to the net positive charge experienced by valence electrons. It increases across a period as the number of protons increases but the shielding effect of inner electrons remains relatively constant.
-
Across a period: The increase in effective nuclear charge leads to a decrease in metallic character. The stronger pull from the nucleus makes it more difficult for the valence electrons to be lost, resulting in less metallic behavior.
-
Down a group: While the effective nuclear charge increases down a group, the effect is lessened by the increased distance of the valence electrons from the nucleus. The added electron shells provide shielding, reducing the effective nuclear charge felt by the outermost electrons, thus contributing to increased metallic character.
Exceptions and Irregularities
While the trends are generally consistent, exceptions and irregularities exist, often stemming from electron configurations and other factors. For instance:
-
Transition metals: Transition metals display a complex interplay of factors affecting their metallic character. While generally metallic, variations in their properties occur due to the filling of d-orbitals, leading to different oxidation states and complex bonding behavior.
-
Lanthanides and Actinides: These elements demonstrate similar complexities, with subtle variations in metallic character due to the filling of f-orbitals.
-
Alkali metals: The alkali metals are highly reactive due to their single valence electron, readily lost to form +1 ions, showcasing strong metallic characteristics.
-
Alkaline earth metals: Similar to alkali metals, but with two valence electrons, resulting in slightly less reactivity and slightly weaker metallic properties compared to the alkali metals.
-
Halogens: These are highly electronegative nonmetals, exhibiting extremely low metallic character.
-
Noble gases: These are inert gases with exceptionally low reactivity and virtually no metallic properties.
Applications and Importance of Metallic Trends
Understanding metallic trends has profound implications across various fields:
1. Material Science
Metallic character is paramount in material science, determining the properties of alloys and compounds. The selection of metals for specific applications depends on their desired mechanical, electrical, and thermal properties, which are directly linked to their metallic character. For instance:
-
Lightweight alloys: Combining metals with low density and high metallic character results in lightweight yet strong materials for aerospace applications.
-
High-strength alloys: Combining metals with high effective nuclear charge and specific electron configurations produces alloys with exceptional strength and durability.
-
Conductive materials: Metals with high electrical and thermal conductivity are crucial in electronics and energy applications.
2. Chemical Reactivity
Metallic character dictates the reactivity of metals. Highly metallic elements readily lose electrons, forming stable ionic compounds, while those with weaker metallic character exhibit varied reactivity. Understanding this helps in predicting reaction outcomes and designing chemical processes.
3. Metallurgy and Extraction
The extraction of metals from their ores depends on their metallic character. More reactive metals require more energy-intensive methods for extraction, while less reactive metals can be obtained through simpler processes.
4. Catalysis
The metallic character of certain elements makes them suitable for use as catalysts in various chemical reactions. Their ability to lose or gain electrons facilitates the reaction process.
Conclusion: A Dynamic Landscape
Metallic trends in the periodic table are not simply a static arrangement but rather a dynamic interplay of atomic structure and properties. Understanding these trends is essential for comprehending a vast range of phenomena in chemistry, material science, and other related fields. The variations and exceptions further highlight the complexities within the periodic table, prompting continuous research and exploration to unravel the nuances of metallic character and its impact on the world around us. Further investigation into specific groups and periods, examining the influence of electron configurations and other factors, will enhance our grasp of this fundamental concept and its wide-ranging applications. The study of metallic character continues to be a vibrant area of research, unveiling new possibilities and driving innovations across numerous industries.
Latest Posts
Latest Posts
-
Lines Of Symmetry On A Trapezoid
Mar 18, 2025
-
Two Same Words With Different Meanings
Mar 18, 2025
-
Select The Correct Statement About Equilibrium
Mar 18, 2025
-
Draw The Major Product Of The Following Reaction
Mar 18, 2025
-
A Wire Loop Of Radius 10 Cm And Resistance
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Metallic Trends In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
