What Is The Molar Mass Of Agno3
News Leon
Mar 17, 2025 · 5 min read
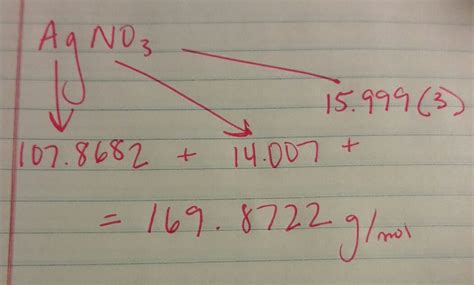
Table of Contents
What is the Molar Mass of AgNO3? A Comprehensive Guide
Determining the molar mass of a compound is a fundamental concept in chemistry, crucial for various calculations and analyses. This comprehensive guide delves into the calculation of the molar mass of silver nitrate (AgNO₃), explaining the underlying principles and providing detailed steps. We'll also explore the importance of molar mass in various chemical applications and address common misconceptions.
Understanding Molar Mass
Before we calculate the molar mass of AgNO₃, let's establish a solid understanding of what molar mass represents. Molar mass is the mass of one mole of a substance. A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³) of particles (atoms, molecules, ions, etc.). Essentially, the molar mass tells us the mass of 6.022 x 10²³ particles of a particular substance in grams.
The molar mass of an element is numerically equal to its atomic weight (or atomic mass) found on the periodic table, expressed in grams per mole (g/mol). For compounds, the molar mass is the sum of the molar masses of all the atoms in the chemical formula.
Calculating the Molar Mass of AgNO₃ (Silver Nitrate)
Silver nitrate (AgNO₃) is an inorganic compound, a salt of silver. To calculate its molar mass, we need the molar masses of its constituent elements: silver (Ag), nitrogen (N), and oxygen (O). These values can be found on the periodic table:
- Silver (Ag): Approximately 107.87 g/mol
- Nitrogen (N): Approximately 14.01 g/mol
- Oxygen (O): Approximately 16.00 g/mol
The chemical formula AgNO₃ indicates that one molecule of silver nitrate contains:
- 1 silver (Ag) atom
- 1 nitrogen (N) atom
- 3 oxygen (O) atoms
Therefore, to calculate the molar mass of AgNO₃, we sum the molar masses of its constituent atoms, considering the number of atoms of each element present:
Molar Mass (AgNO₃) = (1 x Molar Mass of Ag) + (1 x Molar Mass of N) + (3 x Molar Mass of O)
Molar Mass (AgNO₃) = (1 x 107.87 g/mol) + (1 x 14.01 g/mol) + (3 x 16.00 g/mol)
Molar Mass (AgNO₃) = 107.87 g/mol + 14.01 g/mol + 48.00 g/mol
Molar Mass (AgNO₃) = 169.88 g/mol
Therefore, the molar mass of silver nitrate (AgNO₃) is approximately 169.88 g/mol. This means that one mole of AgNO₃ weighs approximately 169.88 grams.
Significance of Molar Mass in Chemical Calculations
The molar mass of AgNO₃, and of any compound, is a fundamental value used extensively in various chemical calculations, including:
1. Mole-to-Mass Conversions:
Knowing the molar mass allows us to convert between the mass of a substance and the number of moles it represents. For example, if we have 10 grams of AgNO₃, we can calculate the number of moles using the following formula:
Number of moles = Mass (g) / Molar Mass (g/mol)
2. Mass-to-Mole Conversions:
Conversely, if we know the number of moles, we can calculate the mass of the substance:
Mass (g) = Number of moles x Molar Mass (g/mol)
3. Stoichiometric Calculations:
Molar mass is essential in stoichiometry, the branch of chemistry dealing with the quantitative relationships between reactants and products in chemical reactions. It enables us to determine the amounts of reactants needed or the amounts of products formed in a chemical reaction.
4. Solution Concentration Calculations:
Molar mass plays a crucial role in calculating the concentration of solutions. For instance, we can determine the molarity (moles of solute per liter of solution) of an AgNO₃ solution if we know the mass of AgNO₃ dissolved in a given volume of solution.
5. Determining Empirical and Molecular Formulas:
Molar mass is vital in determining the empirical and molecular formulas of unknown compounds. Empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula represents the actual number of atoms of each element in a molecule.
Common Misconceptions about Molar Mass
Several common misconceptions surround molar mass:
-
Molar mass is not the same as atomic mass: While numerically similar for elements, they represent different concepts. Atomic mass refers to the average mass of an atom of an element, while molar mass refers to the mass of one mole of a substance.
-
Molar mass is not always a whole number: Due to the average atomic masses of elements, molar mass often involves decimal values.
-
Ignoring significant figures: Always consider the significant figures of the atomic masses when calculating the molar mass to maintain accuracy in subsequent calculations.
Beyond the Basics: Applications of AgNO₃ and its Molar Mass
Silver nitrate (AgNO₃) is a versatile compound with numerous applications, many of which rely on knowing its molar mass for precise calculations:
-
Photography: Historically crucial in photographic processes, AgNO₃ was used to create light-sensitive silver halide crystals. Precise molar mass calculations are important to control the amount of silver halide produced.
-
Medicine: AgNO₃ possesses antimicrobial properties and has been used as an antiseptic in low concentrations. Calculating precise concentrations necessitates the use of molar mass.
-
Chemical Synthesis: AgNO₃ serves as a reagent in various chemical synthesis reactions. Knowing the molar mass is critical in determining stoichiometric ratios and controlling the outcome of reactions.
-
Electroplating: AgNO₃ is used in electroplating to deposit a layer of silver onto objects. Accurate molar mass calculations are necessary for controlling the amount of silver deposited.
-
Analytical Chemistry: In analytical chemistry, AgNO₃ is used in titrations to determine the concentration of halide ions (chlorides, bromides, iodides). Accurate molar mass is essential for precise calculations in volumetric analysis.
Conclusion: The Importance of Precision in Molar Mass Calculations
The accurate determination of the molar mass of AgNO₃, or any compound for that matter, is paramount in various chemical contexts. The procedure detailed above illustrates a straightforward method, but precision is vital. Paying close attention to significant figures, utilizing accurate atomic masses from reliable sources (such as the periodic table), and understanding the underlying concepts of moles and Avogadro's number are critical for successful chemical calculations and a deeper understanding of chemical phenomena. The applications of AgNO₃ highlight the practical implications of understanding and correctly calculating molar mass, demonstrating its importance in fields ranging from medicine and photography to advanced chemical synthesis and analysis. Mastering molar mass calculations is a cornerstone of proficiency in chemistry.
Latest Posts
Latest Posts
-
What Kind Of Acid Is In A Car Battery
Mar 17, 2025
-
What Is The Molar Mass Of Ca Oh 2
Mar 17, 2025
-
Which Of These Structures Is Diploid
Mar 17, 2025
-
How Many Light Years Is Earth From Mars
Mar 17, 2025
-
Serous Membrane That Covers The Lungs
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molar Mass Of Agno3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
