Which Of The Following Compounds Is Most Soluble In Water
News Leon
Mar 31, 2025 · 5 min read
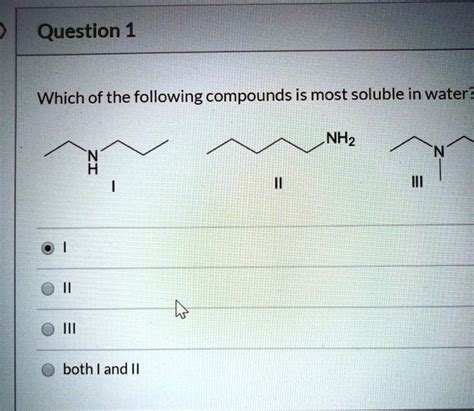
Table of Contents
Which of the Following Compounds is Most Soluble in Water? A Deep Dive into Solubility
Determining the solubility of a compound in water is crucial in various fields, from chemistry and pharmaceuticals to environmental science and geology. Understanding the factors that influence solubility allows us to predict the behavior of substances in aqueous solutions and design effective processes. This article delves into the intricacies of water solubility, exploring the key factors influencing it and providing a framework for comparing the solubility of different compounds. We will then tackle the question posed in the title by considering specific examples and explaining the reasoning behind our conclusions.
Understanding Water Solubility
Solubility, in its simplest form, refers to the maximum amount of a solute that can dissolve in a given amount of solvent at a specific temperature and pressure. For our purposes, the solvent is water (H₂O), a highly polar molecule with a unique ability to dissolve a wide range of substances. However, not all compounds are equally soluble in water. The solubility of a compound is governed by several factors:
1. Polarity and Intermolecular Forces
Water's polarity is the key to understanding its solvent properties. The oxygen atom carries a partial negative charge (δ-), while the hydrogen atoms carry partial positive charges (δ+). This polarity allows water molecules to form strong hydrogen bonds with other polar molecules and ions. The rule of thumb is "like dissolves like." Polar compounds tend to dissolve in polar solvents, while nonpolar compounds dissolve in nonpolar solvents.
-
Polar Compounds: These possess a significant difference in electronegativity between atoms within the molecule, leading to a separation of charge. They readily interact with water's polar nature through dipole-dipole interactions or hydrogen bonding. Examples include sugars, alcohols, and many ionic compounds.
-
Nonpolar Compounds: These have a uniform distribution of charge, resulting in weak intermolecular forces. They struggle to interact with water, leading to low solubility. Examples include hydrocarbons (like oil) and many organic compounds.
2. Hydrogen Bonding
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. Compounds capable of forming hydrogen bonds with water exhibit enhanced solubility. The more hydrogen bonding sites a molecule possesses, the higher its potential solubility in water.
3. Molecular Size and Shape
Larger molecules generally have lower solubility in water. As the size of a nonpolar portion of a molecule increases, its hydrophobic interactions (repulsion from water) become more dominant, reducing solubility. The shape of the molecule also plays a role; a more compact structure often enhances solubility compared to a long, extended chain.
4. Temperature
Temperature significantly influences solubility. For most solid solutes, increasing the temperature generally increases solubility. This is because higher temperatures provide the kinetic energy needed to overcome the intermolecular forces holding the solute together. However, the relationship between temperature and solubility isn't always linear and can be complex for certain substances.
5. Pressure
Pressure primarily affects the solubility of gases in water. According to Henry's Law, the solubility of a gas is directly proportional to its partial pressure above the solution. Increasing the pressure of a gas above a water solution increases the amount of gas that dissolves. The effect of pressure on the solubility of solids and liquids is generally negligible.
Comparing Solubility: A Case Study
Let's consider a hypothetical scenario to illustrate how to compare the solubility of different compounds in water. Imagine we have the following compounds:
- Sodium chloride (NaCl): An ionic compound.
- Ethanol (C₂H₅OH): A polar alcohol.
- Benzene (C₆H₆): A nonpolar hydrocarbon.
- Octanol (C₈H₁₇OH): A larger polar alcohol.
- Sucrose (C₁₂H₂₂O₁₁): A polar sugar.
Based on the principles discussed above, we can rank these compounds in order of decreasing water solubility:
-
Sodium Chloride (NaCl): This is a highly soluble ionic compound. NaCl readily dissociates into Na⁺ and Cl⁻ ions in water, which are strongly hydrated (surrounded by water molecules) due to strong ion-dipole interactions.
-
Sucrose (C₁₂H₂₂O₁₁): This polar sugar contains numerous hydroxyl (-OH) groups, capable of forming numerous hydrogen bonds with water molecules. This extensive hydrogen bonding network leads to high solubility.
-
Ethanol (C₂H₅OH): Ethanol is a polar molecule with a hydroxyl group (-OH) that can participate in hydrogen bonding with water. While it's soluble, its solubility is lower than sucrose and NaCl due to the smaller number of hydrogen bonding sites.
-
Octanol (C₈H₁₇OH): While octanol possesses a hydroxyl group capable of hydrogen bonding, its long hydrocarbon chain is predominantly nonpolar. The hydrophobic interactions of the long carbon chain significantly outweigh the polar interactions of the hydroxyl group, resulting in lower solubility compared to ethanol.
-
Benzene (C₆H₆): Benzene is a nonpolar hydrocarbon with no polar functional groups. It cannot form significant interactions with water molecules, resulting in very low solubility – essentially, it's insoluble in water.
Therefore, in this example, sodium chloride (NaCl) is the most soluble compound in water, followed by sucrose, ethanol, octanol, and lastly, benzene.
Advanced Considerations
The solubility of a compound can be further influenced by other factors including:
-
pH of the solution: The pH affects the ionization state of certain compounds, which impacts their solubility. Weak acids and bases will have different solubility depending on the pH.
-
Presence of other solutes: The presence of other dissolved substances can alter the solubility of a given compound through various mechanisms, such as common ion effects and complexation.
-
Polymorphism: Some compounds exist in different crystalline forms (polymorphs) with different solubilities.
Conclusion
Predicting the solubility of a compound in water requires considering a multitude of factors, primarily polarity, intermolecular forces, molecular size, and temperature. By understanding these fundamental principles, we can effectively analyze and compare the solubility of different compounds and make informed decisions in various applications. The case study presented provides a clear framework for analyzing specific examples. Remember, though, solubility is a complex phenomenon, and the factors influencing it can interact in intricate ways. While the "like dissolves like" principle serves as a good starting point, a comprehensive understanding of intermolecular forces and other contributing factors is essential for accurate prediction.
Latest Posts
Latest Posts
-
Which Pair Of Triangles Must Be Similar
Apr 01, 2025
-
Balanced Equation For Combustion Of Ethane
Apr 01, 2025
-
Every Integer Is A Real Number
Apr 01, 2025
-
Count Vowels In A String Python
Apr 01, 2025
-
Which Of The Following Elements Is Most Electronegative
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Is Most Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
