What Is The Monomer Of Polypeptide
News Leon
Mar 29, 2025 · 7 min read
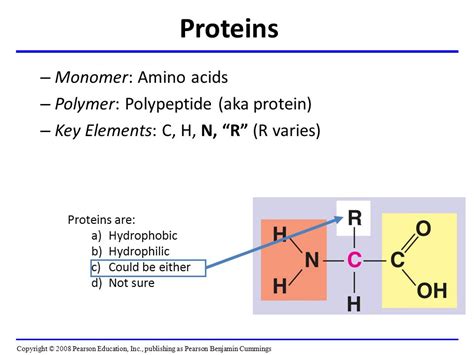
Table of Contents
What is the Monomer of a Polypeptide? Understanding Amino Acids and Peptide Bonds
The fundamental building blocks of life are often complex, yet elegantly structured. Understanding these structures is crucial to grasping the intricate processes that drive biological systems. One such fundamental building block is the polypeptide, a crucial component of proteins. But what exactly is the monomer of a polypeptide? The answer, simply put, is the amino acid. This article will delve deep into the world of amino acids, exploring their structure, properties, and their crucial role in forming polypeptides and ultimately, proteins.
Understanding Amino Acids: The Building Blocks of Polypeptides
Amino acids are organic molecules that serve as the monomers, or individual units, for the creation of polypeptides. They possess a unique structure that dictates their properties and how they interact to form the complex three-dimensional structures of proteins. Each amino acid consists of four main components:
The Four Key Components of an Amino Acid:
-
A Central Carbon Atom (α-carbon): This carbon atom sits at the heart of the amino acid structure, acting as a central point of attachment for the other three components.
-
An Amino Group (-NH₂): This group is characterized by a nitrogen atom bonded to two hydrogen atoms. It is basic and plays a critical role in peptide bond formation.
-
A Carboxyl Group (-COOH): This group is acidic, containing a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-OH). This group also participates in peptide bond formation.
-
A Variable Side Chain (R-group): This is the unique feature that distinguishes one amino acid from another. The R-group varies greatly in structure, size, charge, and polarity, giving each amino acid its specific chemical properties. These properties significantly influence the overall structure and function of the protein it contributes to.
The Diversity of Amino Acids: 20 Essential Building Blocks
There are 20 standard amino acids that are commonly found in proteins. These 20 amino acids are encoded by the genetic code and are used by ribosomes to synthesize polypeptides during protein biosynthesis. The variation in their R-groups is what provides the incredible diversity of protein structures and functions.
Categorizing Amino Acids Based on their R-groups:
Amino acids can be categorized into different groups based on the characteristics of their R-groups:
-
Nonpolar, Aliphatic Amino Acids: These amino acids have hydrocarbon side chains that are hydrophobic (water-repelling). Examples include Glycine (Gly, G), Alanine (Ala, A), Valine (Val, V), Leucine (Leu, L), and Isoleucine (Ile, I).
-
Aromatic Amino Acids: These amino acids possess aromatic ring structures in their side chains. They often absorb UV light and can contribute to protein stability through hydrophobic interactions. Examples include Phenylalanine (Phe, F), Tyrosine (Tyr, Y), and Tryptophan (Trp, W).
-
Polar, Uncharged Amino Acids: These amino acids have side chains that are polar but do not carry a net charge at physiological pH. They can form hydrogen bonds and participate in various interactions within proteins. Examples include Serine (Ser, S), Threonine (Thr, T), Cysteine (Cys, C), Asparagine (Asn, N), and Glutamine (Gln, Q).
-
Positively Charged (Basic) Amino Acids: These amino acids have side chains with a positive charge at physiological pH. They are often involved in ionic interactions within proteins. Examples include Lysine (Lys, K), Arginine (Arg, R), and Histidine (His, H).
-
Negatively Charged (Acidic) Amino Acids: These amino acids have side chains with a negative charge at physiological pH due to carboxyl groups. They also participate in ionic interactions within proteins. Examples include Aspartic acid (Asp, D) and Glutamic acid (Glu, E).
Peptide Bond Formation: Linking Amino Acids to Create Polypeptides
The connection between amino acids to form a polypeptide chain occurs through a process called peptide bond formation. This is a dehydration reaction where the carboxyl group (-COOH) of one amino acid reacts with the amino group (-NH₂) of another amino acid. A molecule of water is released in this process, and a covalent bond is formed between the carbon atom of the carboxyl group and the nitrogen atom of the amino group. This covalent bond is the peptide bond.
The Peptide Bond: A Crucial Covalent Link
The peptide bond possesses a partial double-bond character due to resonance, which restricts rotation around the bond and influences the overall conformation of the polypeptide chain. This partial double bond is significant because it contributes to the rigidity of the peptide backbone and influences the overall three-dimensional structure of the protein.
From Polypeptide to Protein: Levels of Protein Structure
A polypeptide chain, formed by the linkage of multiple amino acids through peptide bonds, is not yet a fully functional protein. The polypeptide needs to fold into a specific three-dimensional structure to become a biologically active protein. Protein structure is organized into four hierarchical levels:
1. Primary Structure: The Amino Acid Sequence
The primary structure refers to the linear sequence of amino acids in the polypeptide chain. This sequence is dictated by the genetic code and is crucial because it determines all subsequent levels of protein structure. Even a single amino acid substitution can drastically alter the protein's function.
2. Secondary Structure: Local Folding Patterns
Secondary structure refers to local folding patterns within the polypeptide chain. Common secondary structures include:
-
α-helices: A coiled structure stabilized by hydrogen bonds between the carbonyl oxygen of one amino acid and the amide hydrogen of another amino acid four residues down the chain.
-
β-sheets: Extended regions of the polypeptide chain arranged side-by-side, stabilized by hydrogen bonds between adjacent strands. These can be parallel or antiparallel depending on the orientation of the strands.
-
Turns and Loops: These are short segments of the polypeptide chain that connect α-helices and β-sheets, contributing to the overall three-dimensional arrangement of the protein.
3. Tertiary Structure: The Overall 3D Arrangement
Tertiary structure refers to the overall three-dimensional arrangement of the polypeptide chain, including the spatial relationships between secondary structure elements. This structure is stabilized by a variety of interactions including:
-
Hydrophobic interactions: Nonpolar side chains cluster together in the protein's interior, away from the surrounding water molecules.
-
Hydrogen bonds: Hydrogen bonds between polar side chains contribute to the stability of the structure.
-
Ionic bonds (salt bridges): Ionic interactions between oppositely charged side chains.
-
Disulfide bonds: Covalent bonds between cysteine residues, further strengthening the protein's structure.
4. Quaternary Structure: Multiple Polypeptide Chains
Quaternary structure applies only to proteins composed of multiple polypeptide chains (subunits). It describes how these subunits assemble to form the complete protein complex. Interactions similar to those stabilizing tertiary structure also maintain the quaternary structure.
The Importance of Polypeptides and Proteins: Biological Roles
Proteins, the ultimate product of polypeptide folding, play a vast array of crucial roles in living organisms. Their diverse functions are a direct result of the diverse properties of amino acids and the resulting complex three-dimensional structures that proteins adopt. Here are just a few examples:
-
Enzymes: Catalyze biochemical reactions.
-
Structural proteins: Provide support and shape to cells and tissues (e.g., collagen, keratin).
-
Transport proteins: Carry molecules across cell membranes (e.g., hemoglobin).
-
Hormones: Act as signaling molecules (e.g., insulin).
-
Antibodies: Part of the immune system, defending against pathogens.
-
Motor proteins: Generate movement (e.g., myosin).
-
Receptor proteins: Bind to signaling molecules and initiate cellular responses.
Conclusion: Amino Acids – The Foundation of Life's Complexity
In summary, the monomer of a polypeptide is the amino acid. The 20 standard amino acids, each with its unique R-group, combine through peptide bonds to form polypeptide chains. These chains then fold into intricate three-dimensional structures, forming proteins – the workhorses of life, responsible for a vast array of crucial biological functions. Understanding the structure and properties of amino acids and the process of peptide bond formation is fundamental to comprehending the complexity and diversity of life at a molecular level. Further exploration into protein structure and function reveals the astonishing elegance and efficiency of biological systems, highlighting the fundamental role of the humble amino acid as the building block of life's most essential molecules.
Latest Posts
Latest Posts
-
A Vector Has Magnitude And Direction
Mar 31, 2025
-
Oxidation Number Of S In So2
Mar 31, 2025
-
Reduction Of An Aldehyde Produces A
Mar 31, 2025
-
Which Of The Following Is An Example Of A Mixture
Mar 31, 2025
-
What Is The Reciprocal Of 1 6
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Monomer Of Polypeptide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
