Oxidation Number Of S In So2
News Leon
Mar 31, 2025 · 5 min read
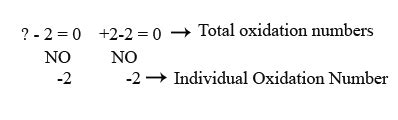
Table of Contents
Determining the Oxidation Number of Sulfur in SO₂: A Comprehensive Guide
Sulfur dioxide (SO₂) is a crucial compound with widespread industrial applications and significant environmental impact. Understanding its chemical properties, particularly the oxidation state of sulfur within the molecule, is fundamental to comprehending its reactivity and behavior. This article delves into the detailed process of calculating the oxidation number of sulfur in SO₂, exploring various approaches and clarifying common misconceptions. We'll also examine the implications of this oxidation number in understanding SO₂'s chemical behavior and its role in environmental chemistry.
Understanding Oxidation Numbers
Before we embark on calculating the oxidation number of sulfur in SO₂, let's establish a clear understanding of the concept itself. Oxidation numbers, also known as oxidation states, are integers assigned to atoms in molecules or ions that represent the hypothetical charge an atom would have if all bonds were completely ionic. This is a crucial distinction; oxidation numbers are not necessarily the actual charges on atoms. They are a bookkeeping tool used to track electron transfer in chemical reactions.
Several rules govern the assignment of oxidation numbers:
-
Rule 1: The oxidation number of an element in its free or uncombined state is zero. For example, the oxidation number of O₂ is 0, and the oxidation number of S₈ is 0.
-
Rule 2: The oxidation number of a monatomic ion is equal to its charge. For example, the oxidation number of Na⁺ is +1, and the oxidation number of Cl⁻ is -1.
-
Rule 3: The oxidation number of hydrogen is usually +1. However, it is -1 in metal hydrides (e.g., NaH).
-
Rule 4: The oxidation number of oxygen is usually -2. However, it is -1 in peroxides (e.g., H₂O₂) and -1/2 in superoxides (e.g., KO₂).
-
Rule 5: The sum of the oxidation numbers of all atoms in a neutral molecule is zero.
-
Rule 6: The sum of the oxidation numbers of all atoms in a polyatomic ion is equal to the charge of the ion.
Calculating the Oxidation Number of Sulfur in SO₂
Now, let's apply these rules to determine the oxidation number of sulfur in SO₂.
-
Identify the elements: The molecule contains sulfur (S) and oxygen (O).
-
Assign oxidation numbers to the known elements: Oxygen typically has an oxidation number of -2 (Rule 4). Since there are two oxygen atoms in SO₂, the total contribution from oxygen is 2 * (-2) = -4.
-
Apply Rule 5: The sum of the oxidation numbers in a neutral molecule (like SO₂) is zero. Therefore, we can set up an equation:
Oxidation number of S + (2 * Oxidation number of O) = 0
-
Solve for the oxidation number of sulfur: Substituting the oxidation number of oxygen (-2), we get:
Oxidation number of S + (2 * -2) = 0 Oxidation number of S - 4 = 0 Oxidation number of S = +4
Therefore, the oxidation number of sulfur in SO₂ is +4.
Alternative Approach: Using Electronegativity
Another way to understand the oxidation number is through the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Oxygen is significantly more electronegative than sulfur. In the SO₂ molecule, the oxygen atoms attract the shared electrons more strongly, resulting in a partial negative charge on the oxygen atoms and a partial positive charge on the sulfur atom. This difference in electronegativity leads to the assignment of the +4 oxidation state to sulfur.
Implications of the +4 Oxidation State of Sulfur in SO₂
The +4 oxidation state of sulfur in SO₂ has significant implications for its chemical reactivity and its role in environmental processes.
Chemical Reactivity:
-
Reducing agent: Sulfur in the +4 oxidation state can be further oxidized to higher oxidation states (+6 for example, in SO₃). This makes SO₂ a good reducing agent, meaning it readily donates electrons to other substances.
-
Oxidizing agent: Conversely, sulfur in SO₂ can be reduced to lower oxidation states (e.g., 0 in elemental sulfur or -2 in sulfides). Therefore, SO₂ can also act as an oxidizing agent under certain conditions.
-
Acid-base reactions: SO₂ dissolves in water to form sulfurous acid (H₂SO₃), a weak acid. This acidic nature is a direct consequence of the sulfur's partial positive charge in the SO₂ molecule.
Environmental Implications:
-
Acid rain: SO₂ is a major precursor to acid rain. When SO₂ is released into the atmosphere, it reacts with water and oxygen to form sulfuric acid (H₂SO₄), which falls as acid rain. This acid rain damages ecosystems, buildings, and infrastructure.
-
Air pollution: SO₂ itself is a significant air pollutant, causing respiratory problems in humans and animals.
-
Ozone depletion: While not as significant as chlorofluorocarbons (CFCs), SO₂ can contribute to ozone depletion in the stratosphere.
Common Misconceptions about Oxidation Numbers
It's important to dispel some common misconceptions surrounding oxidation numbers:
-
Oxidation numbers are not actual charges: As mentioned earlier, oxidation numbers are a bookkeeping tool, not real charges on atoms. While they reflect the electron distribution in a molecule, they are not necessarily the actual charges.
-
Oxidation numbers can be fractional: In some cases, particularly in complex compounds, oxidation numbers can be fractional. This doesn't mean the atom has a fractional charge; it simply reflects the average oxidation state of multiple atoms of the same element in the molecule.
-
Oxidation numbers change during redox reactions: Oxidation numbers are a useful tool for tracking electron transfer in redox reactions (reduction-oxidation reactions). An increase in oxidation number indicates oxidation (loss of electrons), while a decrease indicates reduction (gain of electrons).
Conclusion
Determining the oxidation number of sulfur in SO₂ is a straightforward application of fundamental chemical principles. The +4 oxidation state of sulfur dictates its significant role in both chemical reactions and environmental processes. Understanding this oxidation number provides valuable insight into the reactivity and environmental impact of sulfur dioxide, emphasizing the importance of understanding fundamental chemical concepts to tackle complex environmental challenges. The ability to correctly calculate oxidation numbers is a fundamental skill for any student or professional working in chemistry or related fields. This detailed explanation, including the clarification of common misconceptions, should solidify understanding and provide a strong foundation for further exploration of redox chemistry.
Latest Posts
Latest Posts
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
-
The Slope Of Speed Time Graph Indicates
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of S In So2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
