Which Of The Following Is An Example Of A Mixture
News Leon
Mar 31, 2025 · 5 min read
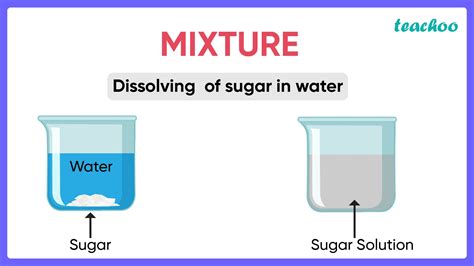
Table of Contents
Which of the Following is an Example of a Mixture? A Deep Dive into Mixtures and Their Types
The question, "Which of the following is an example of a mixture?" might seem simple at first glance. However, understanding the nuances of mixtures requires delving into the fundamental concepts of chemistry and the diverse ways substances can combine. This comprehensive guide will not only answer this question but also provide a thorough exploration of mixtures, their classifications, properties, and real-world examples. We'll examine various types of mixtures and help you confidently identify them.
What is a Mixture?
A mixture is a substance composed of two or more components that are not chemically bonded. This means the components retain their individual chemical properties and can be separated by physical methods, such as filtration, distillation, evaporation, or chromatography. Crucially, the proportions of the components in a mixture can vary. Unlike compounds, where the elements are chemically bound in fixed ratios, the composition of a mixture is not fixed.
Key Characteristics of Mixtures:
- Variable composition: The ratio of components in a mixture can change.
- Retention of individual properties: The components retain their original properties.
- Separation by physical means: Components can be separated using physical methods.
- No chemical reaction: The components do not react chemically with each other.
Types of Mixtures:
Mixtures are broadly classified into two main categories: homogeneous and heterogeneous. Let's examine each in detail.
Homogeneous Mixtures:
A homogeneous mixture is one where the components are uniformly distributed throughout the mixture. At a macroscopic level, it appears to be a single substance. The composition is consistent throughout the sample.
Examples of Homogeneous Mixtures:
- Saltwater: Salt dissolves completely in water, forming a uniform solution.
- Air: A mixture of gases (nitrogen, oxygen, argon, etc.) uniformly distributed.
- Brass: An alloy of copper and zinc, with a uniform composition.
- Sugar dissolved in water: The sugar molecules are evenly dispersed in the water.
- Vinegar: A homogeneous solution of acetic acid and water.
- Steel: An alloy of iron and carbon, with consistent properties throughout.
- Gasoline: A blend of various hydrocarbons, creating a uniform liquid.
Key Characteristics of Homogeneous Mixtures:
- Uniform composition: The components are evenly distributed.
- Single phase: The mixture exists in a single phase (solid, liquid, or gas).
- Particles are invisible to the naked eye: The components are thoroughly mixed and not easily distinguishable.
Heterogeneous Mixtures:
A heterogeneous mixture is one where the components are not uniformly distributed. Different parts of the mixture have different compositions and properties. The different components are easily visible.
Examples of Heterogeneous Mixtures:
- Sand and water: Sand particles are clearly visible and do not dissolve in water.
- Oil and water: Oil and water do not mix, forming distinct layers.
- A salad: A mixture of various vegetables and ingredients that retain their individual identities.
- Granite: A rock composed of visible crystals of different minerals.
- Soil: A mixture of various organic and inorganic materials, with varying particle sizes.
- Pizza: A mixture of various ingredients, including cheese, sauce, and toppings.
- Concrete: A mixture of cement, aggregate (sand and gravel), and water.
Key Characteristics of Heterogeneous Mixtures:
- Non-uniform composition: The components are not evenly distributed.
- Multiple phases: The mixture may exist in multiple phases (solid, liquid, gas).
- Particles are visible to the naked eye: The components are easily distinguishable.
Solutions, Suspensions, and Colloids: Sub-categories of Mixtures
Within the broader categories of homogeneous and heterogeneous mixtures, we can further classify mixtures based on particle size and other properties:
-
Solutions: These are homogeneous mixtures where one substance (the solute) is dissolved completely in another (the solvent). The particles of the solute are very small (ions or molecules) and are not visible to the naked eye. Examples include saltwater, sugar water, and air.
-
Suspensions: These are heterogeneous mixtures where particles of one substance are dispersed throughout another, but these particles are large enough to settle out over time. Examples include muddy water, where the mud particles eventually settle to the bottom, or a mixture of sand and water. The particles are visible to the naked eye.
-
Colloids: These are mixtures that fall between solutions and suspensions. The dispersed particles are larger than in solutions but smaller than in suspensions. They do not settle out readily. Examples include milk (fat globules dispersed in water), fog (water droplets dispersed in air), and mayonnaise (oil droplets dispersed in water). The particles are often not visible to the naked eye, but they can scatter light (Tyndall effect).
Identifying Mixtures: Practical Examples
To solidify your understanding, let's consider some examples and determine whether they are mixtures and, if so, what type:
-
A glass of orange juice: This is a heterogeneous mixture (unless it's been strained). It contains pulp (solid particles) and liquid.
-
Stainless steel: This is a homogeneous mixture (alloy) of iron, chromium, and nickel.
-
Chocolate chip cookies: This is a heterogeneous mixture. The chocolate chips are clearly distinguishable from the cookie dough.
-
Seawater: This is primarily a homogeneous mixture of water and dissolved salts. However, depending on the location and conditions, it may also contain suspended particles (heterogeneous components).
-
Blood: Blood is a complex mixture containing several components. It can be considered a heterogeneous mixture because different components like red blood cells, white blood cells, and plasma are distinguishable. It is also considered a colloid due to the presence of proteins and other large molecules.
The Importance of Understanding Mixtures
Understanding the different types of mixtures is crucial in various fields:
- Chemistry: The concept of mixtures forms the basis of many chemical processes and separations.
- Environmental science: Analyzing the composition of mixtures in air and water is essential for monitoring pollution levels.
- Materials science: Creating new materials often involves mixing different substances with specific properties.
- Food science: The properties of food products depend on the types of mixtures used in their preparation.
- Medicine: Understanding mixtures is essential in pharmaceutical preparations and drug delivery systems.
Conclusion:
Determining whether a substance is a mixture hinges on understanding the key characteristics of mixtures: variable composition, retention of individual properties, and separability by physical methods. Furthermore, differentiating between homogeneous and heterogeneous mixtures requires careful observation of the uniformity of the components' distribution. By grasping these concepts and exploring the diverse examples provided, you can confidently identify mixtures and appreciate their significance in various scientific and everyday contexts. Remember that the world around us is largely composed of mixtures, from the air we breathe to the food we eat, and understanding their properties is essential for comprehending our environment.
Latest Posts
Latest Posts
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
-
The Slope Of Speed Time Graph Indicates
Apr 02, 2025
-
What Is The Approximate Size Of A Nucleus
Apr 02, 2025
-
Hydrogen Peroxide Catalyzed By Manganese Dioxide
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Example Of A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
