Reduction Of An Aldehyde Produces A
News Leon
Mar 31, 2025 · 5 min read
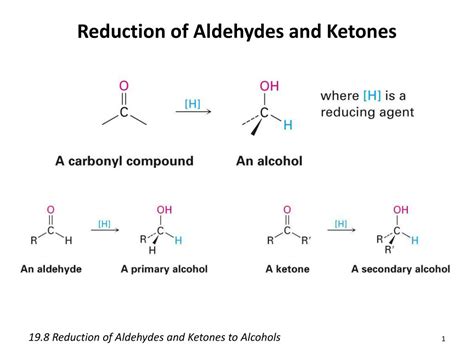
Table of Contents
Reduction of an Aldehyde Produces a Primary Alcohol: A Comprehensive Guide
The reduction of an aldehyde is a fundamental organic chemistry reaction with widespread applications in various fields, including pharmaceuticals, materials science, and industrial chemistry. Understanding this reaction mechanism and its implications is crucial for any aspiring chemist. This article will delve deep into the reduction of aldehydes, exploring different reducing agents, reaction mechanisms, and the resulting product: a primary alcohol. We'll also touch upon the practical aspects and applications of this transformation.
Understanding Aldehydes and Their Reactivity
Aldehydes are organic compounds containing a carbonyl group (C=O) bonded to at least one hydrogen atom. This structural feature is responsible for their unique reactivity. The carbonyl carbon, being electrophilic (electron-deficient), is susceptible to nucleophilic attack. This characteristic is central to the reduction process. The presence of the hydrogen atom differentiates aldehydes from ketones, which possess two alkyl or aryl groups attached to the carbonyl carbon. This difference in structure dictates the type of alcohol produced upon reduction.
The Carbonyl Carbon: A Site of Reactivity
The polarized nature of the carbonyl group is key. The oxygen atom is more electronegative than the carbon atom, leading to a partial positive charge (δ+) on the carbon and a partial negative charge (δ-) on the oxygen. This makes the carbonyl carbon a prime target for nucleophiles, which are electron-rich species seeking positively charged centers. This nucleophilic attack is the cornerstone of many aldehyde reactions, including reduction.
Reduction of Aldehydes: The Path to Primary Alcohols
The reduction of an aldehyde involves the addition of two hydrogen atoms (H₂) to the carbonyl group. This process converts the carbonyl group (C=O) into a hydroxyl group (-OH), resulting in the formation of a primary alcohol. This reaction typically requires a reducing agent, a chemical species capable of donating electrons. Several different reducing agents can achieve this transformation, each possessing its own advantages and disadvantages.
Common Reducing Agents for Aldehyde Reduction
Several reagents are effective in reducing aldehydes to primary alcohols. Each has unique properties that make it suitable for specific applications. Here are some of the most commonly used:
1. Sodium Borohydride (NaBH₄): This is a mild reducing agent that selectively reduces aldehydes and ketones. It's commonly used in aprotic solvents (like ethanol or methanol) to avoid unwanted side reactions. Its mildness makes it suitable for substrates sensitive to harsher reducing agents. The reaction proceeds via a nucleophilic hydride (H⁻) attack on the carbonyl carbon.
2. Lithium Aluminum Hydride (LiAlH₄): A much stronger reducing agent than NaBH₄, LiAlH₄ reduces not only aldehydes and ketones but also esters, carboxylic acids, and even nitriles. This powerful reducing agent is typically used in anhydrous ether solvents. Its strength necessitates careful handling due to its reactivity with water. It also proceeds through a nucleophilic hydride attack.
3. Catalytic Hydrogenation: This method uses hydrogen gas (H₂) in the presence of a metal catalyst (e.g., platinum, palladium, or nickel) to reduce the aldehyde. This is a gentler method compared to LiAlH₄ but may require higher pressures and temperatures depending on the substrate.
Reaction Mechanisms: A Detailed Look
The general mechanism for aldehyde reduction involves nucleophilic attack by the hydride ion (H⁻) from the reducing agent. This attack occurs on the electrophilic carbonyl carbon, forming a tetrahedral intermediate. Subsequent protonation of the alkoxide ion yields the primary alcohol.
Mechanism using NaBH₄:
-
Nucleophilic Attack: The hydride ion from NaBH₄ attacks the electrophilic carbonyl carbon of the aldehyde.
-
Tetrahedral Intermediate Formation: This leads to the formation of a tetrahedral intermediate, where the oxygen carries a negative charge.
-
Protonation: A proton source (e.g., water or alcohol) protonates the negatively charged oxygen, generating the primary alcohol.
-
Regeneration of the Reducing Agent: The borate byproduct is formed.
Mechanism using LiAlH₄: Similar steps are involved, but LiAlH₄ is a more powerful reducing agent due to the greater nucleophilicity of the hydride ion bound to aluminum.
Factors Affecting Aldehyde Reduction
Several factors can influence the efficiency and selectivity of aldehyde reduction:
-
Solvent: The choice of solvent is crucial. Protic solvents can participate in the reaction, affecting the outcome.
-
Temperature: Reaction temperature influences the rate and selectivity. Higher temperatures can lead to side reactions.
-
Steric Hindrance: Bulky groups around the carbonyl group can hinder the approach of the reducing agent, affecting the reaction rate.
-
Reducing Agent Choice: The selection of the reducing agent depends on the substrate's sensitivity and the desired outcome. NaBH₄ is suitable for sensitive substrates, while LiAlH₄ is necessary for less reactive ones.
Applications of Aldehyde Reduction
The reduction of aldehydes to primary alcohols finds extensive applications across various chemical domains:
-
Pharmaceutical Industry: Many pharmaceutical compounds contain alcohol functional groups, which are often synthesized through the reduction of aldehydes.
-
Fine Chemicals Synthesis: The synthesis of numerous fine chemicals, perfumes, and flavors often involves this reaction.
-
Polymer Chemistry: Aldehyde reduction can be employed to modify polymers and improve their properties.
-
Industrial Chemistry: Large-scale production of various chemicals often relies on this fundamental reaction.
Conclusion: A Versatile and Essential Reaction
The reduction of an aldehyde to a primary alcohol is a cornerstone reaction in organic chemistry. Understanding the mechanism, the various reducing agents, and the factors influencing the reaction is critical for anyone working in chemical synthesis. The versatility of this reaction, coupled with its widespread applications across various industries, highlights its importance in modern chemistry. The choice of reducing agent depends on factors like substrate sensitivity and reaction conditions, making it a highly adaptable method for producing valuable primary alcohols. Continued research and development in this area will undoubtedly lead to further improvements and applications of this fundamental organic transformation.
Latest Posts
Latest Posts
-
What Is The Difference Between A Primary And Secondary Consumer
Apr 02, 2025
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Reduction Of An Aldehyde Produces A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
