The Noble Gases Are Also Called The
News Leon
Mar 23, 2025 · 5 min read
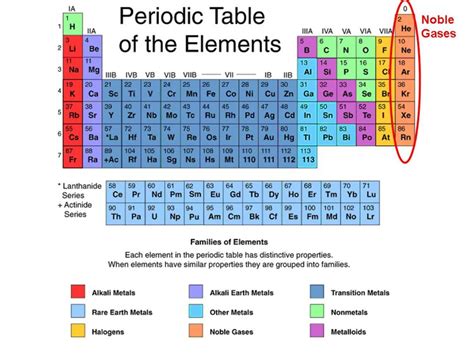
Table of Contents
The Noble Gases Are Also Called the Inert Gases: A Deep Dive into Their Properties and Applications
The noble gases, also known as inert gases, are a unique group of elements residing in Group 18 of the periodic table. Their unique properties, stemming from their complete valence electron shells, have led to a wide array of applications across various industries. Understanding their characteristics, history of discovery, and uses is crucial to appreciating their significance in modern science and technology. This comprehensive exploration delves deep into the world of noble gases, clarifying their alternative name and unraveling the science behind their remarkable behavior.
Why "Inert Gases"? Understanding the Name
The term "inert gases" stems from the historical observation that these elements were exceptionally unreactive. For a long time, they were believed to be completely incapable of forming chemical compounds, hence the designation "inert," meaning inactive or sluggish. This perception, however, has been revised significantly with advancements in chemical synthesis. While their reactivity is indeed low, it's not entirely absent. Under specific conditions, some noble gases can participate in chemical reactions, forming compounds, albeit often under extreme conditions or with highly reactive species. This reactivity, though limited, necessitates a nuanced understanding of the term "inert," which is why the term "noble gases" is increasingly favored.
The "Noble" Gases: A More Accurate Description
The term "noble gases" reflects the elements' relative unwillingness to participate in chemical reactions. Similar to noble metals like gold and platinum, which are resistant to corrosion and chemical change, noble gases exhibit a remarkable stability due to their complete outer electron shells (octet rule). This filled valence shell provides exceptional stability, minimizing their tendency to gain, lose, or share electrons with other atoms. This inherent stability is what makes them truly unique within the periodic table.
Key Properties of Noble Gases
Several properties distinguish noble gases from other elements:
1. Complete Valence Electron Shells:
The defining characteristic of noble gases is their complete outermost electron shell. This stable electronic configuration makes them exceptionally unreactive. Helium, with two electrons in its outer shell (1s²), is an exception, but it still exhibits remarkable stability.
2. Low Boiling Points:
Noble gases exist as monatomic gases under standard conditions, meaning they exist as single atoms rather than molecules. The weak interatomic forces between these atoms lead to exceptionally low boiling points. Helium possesses the lowest boiling point of any element.
3. Colorless, Odorless, and Tasteless:
In their pure forms, noble gases are colorless, odorless, and tasteless. This lack of sensory properties contributes to their safe handling in certain applications.
4. Low Reactivity:
While not completely inert, their low reactivity stems from their stable electron configuration. The energy required to disrupt this stability is significantly high, thus limiting their participation in chemical reactions.
5. Poor Conductors of Electricity and Heat:
Noble gases are generally poor conductors of electricity and heat in their gaseous state. This property is crucial for their use in certain types of lighting and insulation.
The Noble Gases: A Detailed Look at Each Element
Let's examine each noble gas individually, highlighting their unique properties and applications:
Helium (He):
Helium, the second lightest element, is known for its low density and incredibly low boiling point. Its uses range from filling balloons and blimps (lighter than air) to cryogenics (cooling systems for MRI machines and scientific research).
Neon (Ne):
Neon is famous for its vibrant orange-red glow in neon signs. It's also used in lasers and some types of high-voltage indicators.
Argon (Ar):
Argon is the most abundant noble gas in Earth's atmosphere and is widely used as an inert atmosphere in welding and metallurgical processes to prevent oxidation.
Krypton (Kr):
Krypton finds applications in high-intensity lighting, such as in certain types of photographic flashes and high-power lasers.
Xenon (Xe):
Xenon is employed in high-intensity arc lamps and specialized lighting applications. It has also seen use in some medical applications.
Radon (Rn):
Radon is a radioactive gas, unlike the other noble gases. Its radioactive properties make it a potential health hazard, and it's not used in widespread applications.
Applications of Noble Gases
The unique properties of noble gases translate into a diverse range of applications:
1. Lighting:
Neon signs, fluorescent lamps, and high-intensity arc lamps are some examples where noble gases find extensive use. Their ability to emit light when electrically excited makes them essential components in lighting technologies.
2. Welding and Metallurgy:
Argon, in particular, plays a critical role as a shielding gas in welding processes. It prevents oxidation and contamination of the weld joint.
3. Cryogenics:
Helium's extremely low boiling point makes it indispensable in cryogenic applications, allowing for the attainment of extremely low temperatures required for various scientific and medical procedures.
4. Medical Applications:
Helium is used in MRI machines, while xenon has seen some medical applications, though less widespread compared to other gases.
5. Scientific Research:
Noble gases are invaluable in various scientific research areas, including analytical chemistry, spectroscopy, and material science.
6. Electronics:
Certain noble gases are used in electronics manufacturing, providing inert atmospheres during sensitive fabrication processes.
7. Nuclear Power Plants:
Some noble gases are used in nuclear power plants for various safety and control systems.
The Ongoing Evolution of Our Understanding of Noble Gas Reactivity
The long-held belief in the complete inertness of noble gases has been challenged and significantly refined. While their reactivity is indeed low, it's not zero. Scientists have successfully synthesized compounds containing xenon, krypton, and even radon under specific conditions. These discoveries showcase the ever-evolving nature of scientific understanding and highlight that even elements once considered completely unreactive can display surprising behavior under carefully controlled circumstances.
Conclusion: A Legacy of Stability and Versatility
The noble gases, whether called "inert" or "noble," represent a fascinating group of elements whose properties have revolutionized various industries and continue to inspire scientific curiosity. Their low reactivity, coupled with their unique physical properties, has led to widespread applications in lighting, medicine, industrial processes, and scientific research. The ongoing exploration of their reactivity and potential for new applications ensures that these gases will remain a significant area of scientific investigation for years to come. The accurate and more encompassing term, "noble gases," reflects their unique position in the periodic table and their remarkable versatility, highlighting their importance in both historical and modern scientific contexts. The evolution of our understanding from "inert" to "noble" reflects the ever-expanding knowledge of the intricate relationships between atoms and their remarkable potential.
Latest Posts
Latest Posts
-
How Is A Map Different From A Globe
Mar 24, 2025
-
Which Of The Following Statements Is A Contradiction
Mar 24, 2025
-
How Many Neutrons Does Sr Have
Mar 24, 2025
-
Which Group In The Periodic Table Contains Only Metals
Mar 24, 2025
-
Provincial Governor Of The Mogul Empire
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about The Noble Gases Are Also Called The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
