Which Group In The Periodic Table Contains Only Metals
News Leon
Mar 24, 2025 · 5 min read
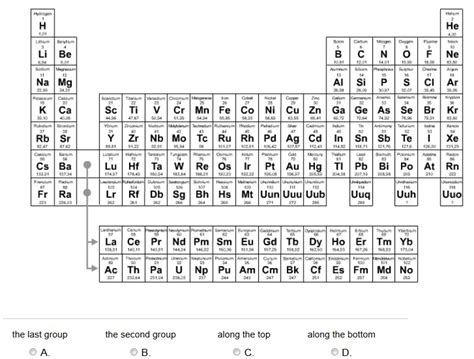
Table of Contents
Which Group in the Periodic Table Contains Only Metals?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and resulting properties. While many groups exhibit a mix of metallic and non-metallic characteristics, one stands out for its unwavering commitment to metallic behavior: Group 1, also known as the alkali metals. This article delves deep into why this is the case, exploring the unique electronic configurations, physical and chemical properties, and the implications of their consistent metallic nature.
The Defining Characteristics of Metals
Before focusing on Group 1, let's establish what defines a metal. Metals are generally characterized by several key properties:
- High electrical conductivity: Metals readily conduct electricity due to the presence of freely moving electrons in their structure, often referred to as a "sea" of delocalized electrons.
- High thermal conductivity: Similar to electrical conductivity, metals efficiently transfer heat due to these mobile electrons.
- Malleability and ductility: Metals can be hammered into sheets (malleability) and drawn into wires (ductility) without breaking, a testament to their ability to deform without fracturing.
- Luster: Metals typically possess a shiny appearance, reflecting light effectively.
- High density: Generally, metals have a high density compared to non-metals. This is a consequence of the close packing of atoms in their metallic structures.
- Low ionization energies: Metals readily lose electrons, forming positive ions (cations). This is a key factor driving their reactivity and bonding behavior.
Group 1: The Alkali Metals – A Deep Dive
Group 1 elements—lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr)—are all alkali metals. The term "alkali" refers to their oxides and hydroxides forming strongly alkaline (basic) solutions in water. Their consistent metallic behavior is a direct consequence of their electronic configuration:
Electronic Configuration and Metallic Bonding
Alkali metals have a single electron in their outermost shell (valence shell). This single valence electron is relatively loosely held, readily participating in metallic bonding. In metallic bonding, valence electrons are delocalized, forming a "sea" of electrons that surrounds positively charged metal ions. This "sea" of electrons enables the high electrical and thermal conductivity characteristic of metals. The weak attraction between the nucleus and the single valence electron also explains their low ionization energies and high reactivity.
Physical Properties: A Consistent Metallic Theme
The physical properties of alkali metals reinforce their consistently metallic nature:
- Low melting and boiling points: Compared to other metals, alkali metals have relatively low melting and boiling points. This is because the metallic bonding is relatively weak due to the presence of only one valence electron per atom. The strength of metallic bonding increases with the number of valence electrons.
- Softness: Alkali metals are very soft and can be easily cut with a knife. This softness reflects the weak metallic bonding and the ease with which metal ions can slide past each other.
- Low density: While still denser than non-metals, alkali metals have relatively low densities compared to other metals, again a consequence of the weaker metallic bonding leading to a less compact structure.
- Silvery-white appearance (except for cesium which has a golden hue): This luster is a hallmark of metallic character, resulting from the interaction of light with the delocalized electrons.
Chemical Properties: High Reactivity, A Metallic Trait
The chemical properties of alkali metals are overwhelmingly driven by their strong tendency to lose their single valence electron, forming a +1 cation. This reactivity increases down the group as the atomic radius increases, and the outermost electron becomes further from the nucleus and easier to remove.
- Reaction with water: This is perhaps the most dramatic demonstration of their reactivity. Alkali metals react violently with water, producing hydrogen gas and a strongly alkaline solution. The reaction becomes progressively more vigorous as you move down the group.
- Reaction with halogens: Alkali metals readily react with halogens (Group 17) to form ionic compounds (salts). These reactions are highly exothermic, releasing significant amounts of heat.
- Reaction with oxygen: Alkali metals react with oxygen to form oxides. The nature of the oxide varies depending on the specific alkali metal and the reaction conditions.
- Reaction with acids: Alkali metals react vigorously with acids, producing hydrogen gas and a salt.
Exceptions and Nuances
While all alkali metals are definitively metallic, some nuances exist:
- Francium: Being highly radioactive and having an extremely short half-life, francium's properties are less extensively studied than the other alkali metals. However, its electronic configuration dictates its metallic nature.
- Lithium: While sharing the general properties of alkali metals, lithium displays some unique behavior due to its small size and high charge density. For instance, it forms stronger bonds compared to heavier alkali metals.
Contrasting Group 1 with Other Groups
To further emphasize the unique position of Group 1, let's contrast it with other groups:
- Group 2 (Alkaline Earth Metals): While also metals, alkaline earth metals have two valence electrons, leading to stronger metallic bonding and different chemical properties than alkali metals. They are less reactive than alkali metals.
- Transition Metals (Groups 3-12): Transition metals have partially filled d orbitals, influencing their properties significantly. They exhibit a wider range of oxidation states and complex ion formation, distinct from alkali metals.
- Groups 13-16: These groups contain a mix of metals, metalloids, and non-metals, highlighting the gradual transition in properties across the periodic table. The metallic character decreases as you move to the right across the periodic table.
- Group 17 (Halogens): These are non-metals, strongly electronegative, readily gaining electrons to form negative ions (anions), the opposite behavior to alkali metals.
- Group 18 (Noble Gases): These are inert gases with full valence shells, making them extremely unreactive and completely lacking metallic properties.
Conclusion: The Undisputed Metallic Reign of Group 1
In conclusion, Group 1, the alkali metals, represents the only group in the periodic table containing exclusively metals. Their consistent metallic behavior stems from their electronic configuration with a single valence electron, leading to weak but effective metallic bonding. Their characteristic physical and chemical properties, ranging from softness and low melting points to high reactivity with water and halogens, all stem from this fundamental electronic structure. While subtle variations exist among the alkali metals, their shared metallic nature remains undeniable, firmly establishing their position as the quintessential example of a group composed entirely of metals. Understanding the electronic configuration and resulting properties of Group 1 provides a crucial foundation for comprehending the periodic trends and the nature of metallic bonding.
Latest Posts
Latest Posts
-
What Is The Function Of Areolar Tissue
Mar 28, 2025
-
What Is 6 25 As A Fraction
Mar 28, 2025
-
Which Of The Following Compounds Is Ionic
Mar 28, 2025
-
Which Characteristic Is Common To All Chordates
Mar 28, 2025
-
Give The Major Product For The Following Reaction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Group In The Periodic Table Contains Only Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
