Oxidation Number Of Cr In Cr2o72-
News Leon
Mar 22, 2025 · 5 min read
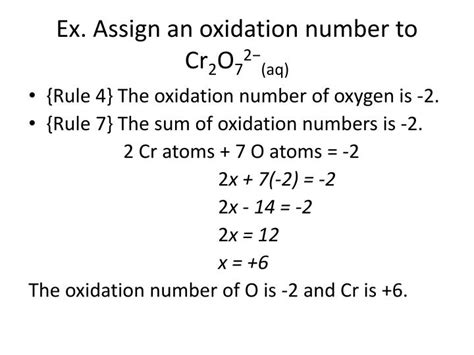
Table of Contents
Determining the Oxidation Number of Cr in Cr₂O₇²⁻: A Comprehensive Guide
The dichromate ion, Cr₂O₇²⁻, is a powerful oxidizing agent frequently encountered in chemistry, particularly in redox reactions. Understanding its structure and, importantly, the oxidation number of chromium (Cr) within the ion is crucial for predicting its reactivity and balancing redox equations. This comprehensive guide will delve into the process of determining the oxidation number of Cr in Cr₂O₇²⁻, exploring the underlying principles and providing a step-by-step explanation.
Understanding Oxidation Numbers
Before we tackle the specific case of Cr₂O₇²⁻, let's establish a solid foundation in the concept of oxidation numbers. The oxidation number, also known as the oxidation state, represents the hypothetical charge an atom would have if all bonds to atoms of different elements were 100% ionic. It's a valuable tool for:
- Balancing redox reactions: Accurately determining oxidation numbers is essential for correctly balancing complex redox equations.
- Predicting reactivity: Oxidation numbers provide insights into an atom's tendency to gain or lose electrons, thus influencing its chemical behavior.
- Understanding chemical bonding: Oxidation numbers offer a simplified view of charge distribution within molecules and ions.
Several rules govern the assignment of oxidation numbers:
- Free elements: The oxidation number of an atom in its elemental form is always zero (e.g., O₂ has an oxidation number of 0 for each oxygen atom).
- Monatomic ions: The oxidation number of a monatomic ion is equal to its charge (e.g., Na⁺ has an oxidation number of +1).
- Hydrogen: Hydrogen typically has an oxidation number of +1, except in metal hydrides (e.g., NaH), where it's -1.
- Oxygen: Oxygen usually has an oxidation number of -2, except in peroxides (e.g., H₂O₂) where it's -1 and in superoxides where it's -1/2.
- Fluorine: Fluorine always has an oxidation number of -1.
- The sum of oxidation numbers: In a neutral molecule, the sum of the oxidation numbers of all atoms must be zero. In a polyatomic ion, the sum of the oxidation numbers must equal the charge of the ion.
Calculating the Oxidation Number of Cr in Cr₂O₇²⁻
Now, let's apply these rules to determine the oxidation number of chromium (Cr) in the dichromate ion, Cr₂O₇²⁻.
Step 1: Identify the known oxidation numbers.
We know the oxidation number of oxygen (O) is typically -2. Since we have seven oxygen atoms, their total contribution to the overall charge is 7 * (-2) = -14.
Step 2: Set up an algebraic equation.
Let 'x' represent the oxidation number of each chromium atom. Since there are two chromium atoms, their total contribution is 2x.
The sum of the oxidation numbers must equal the overall charge of the ion, which is -2. Therefore, we can set up the following equation:
2x + (-14) = -2
Step 3: Solve for x.
Adding 14 to both sides of the equation, we get:
2x = +12
Dividing both sides by 2, we find:
x = +6
Therefore, the oxidation number of chromium (Cr) in Cr₂O₇²⁻ is +6.
Implications of the +6 Oxidation State
The +6 oxidation state of chromium in the dichromate ion is significant for several reasons:
-
Oxidizing power: Cr(VI) is a strong oxidizing agent. This means it readily accepts electrons from other substances, undergoing reduction to a lower oxidation state (e.g., Cr³⁺). This property makes dichromate solutions widely used in various chemical applications, including titrations and organic synthesis.
-
Color: The intense orange color of dichromate solutions is a characteristic of Cr(VI) compounds. The electronic configuration of Cr(VI) leads to strong absorption of light in the blue-green region of the spectrum, resulting in the complementary orange color.
-
Toxicity: Cr(VI) compounds are known to be toxic and carcinogenic. Appropriate safety precautions must always be taken when handling dichromate solutions.
Further Applications and Considerations
The principles discussed here extend beyond the dichromate ion. Determining oxidation numbers is a fundamental skill in chemistry, applicable to a wide range of compounds and ions. This skill is particularly crucial when:
-
Balancing redox reactions: The half-reaction method requires a precise understanding of oxidation number changes to correctly balance electrons transferred during oxidation and reduction. For example, in the reaction of dichromate with iron(II) ions, balancing the equation relies on knowing that Cr(VI) is reduced to Cr(III) while Fe(II) is oxidized to Fe(III).
-
Predicting reaction spontaneity: The change in oxidation numbers indicates the transfer of electrons, and the magnitude of this change is directly related to the potential difference (voltage) in a redox reaction. This allows for the prediction of reaction spontaneity using electrochemical data.
-
Nomenclature: The oxidation number of an element influences its naming in chemical compounds. For example, chromium(III) oxide distinguishes it from other chromium oxides with different oxidation states.
-
Analyzing complex reactions: Many complex reactions involve multiple oxidation-reduction steps. Assigning oxidation numbers to each element in the reactants and products helps to understand the reaction mechanism and the changes occurring during the process.
Beyond Simple Rules: Exceptions and Complex Cases
While the rules mentioned earlier are generally applicable, exceptions exist, especially in complex organometallic compounds and compounds with unusual bonding. In these cases, a more nuanced approach may be needed, which may require the use of advanced spectroscopic techniques and computational chemistry to determine the actual charge distribution and oxidation states. However, even in these cases, the understanding of basic oxidation number principles is a fundamental starting point.
Conclusion
Determining the oxidation number of Cr in Cr₂O₇²⁻ is a straightforward yet crucial exercise that highlights the importance of oxidation numbers in understanding chemical reactivity and structure. The +6 oxidation state of chromium in this ion is a key factor in its oxidizing ability, color, and toxicity. Mastering the principles of assigning oxidation numbers is essential for success in various aspects of chemistry, from balancing equations to understanding complex reaction mechanisms. By understanding the underlying principles and applying a systematic approach, you can confidently determine oxidation numbers for a wide range of compounds and ions, enhancing your understanding of chemical behavior. Remember, while simple rules serve as a good starting point, more complex scenarios may require advanced techniques and analysis. However, a firm grasp of the fundamentals ensures a strong foundation for tackling more intricate chemical problems.
Latest Posts
Latest Posts
-
Thymine Is Replaced By Which Nitrogen Base In Rna
Mar 23, 2025
-
Which Of The Following Bones Belong To The Axial Skeleton
Mar 23, 2025
-
The Noble Gases Are Also Called The
Mar 23, 2025
-
Number Of Valence Electrons In Magnesium
Mar 23, 2025
-
Which Of The Following Is An Aldohexose
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Oxidation Number Of Cr In Cr2o72- . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
