Is C Or N More Electronegative
News Leon
Mar 26, 2025 · 6 min read
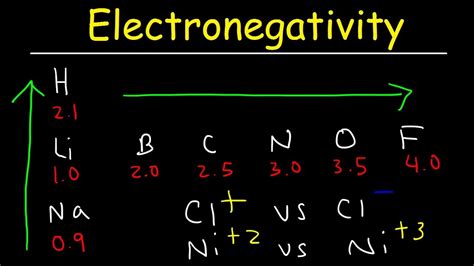
Table of Contents
Is C or N More Electronegative? A Deep Dive into Electronegativity
Electronegativity, a fundamental concept in chemistry, dictates how strongly an atom attracts electrons within a chemical bond. Understanding electronegativity differences is crucial for predicting the polarity of bonds, the geometry of molecules, and their overall reactivity. This article delves into the electronegativity of carbon (C) and nitrogen (N), comparing their values, exploring the underlying reasons for the difference, and showcasing the implications of this difference in various chemical contexts.
Understanding Electronegativity
Before comparing carbon and nitrogen, let's establish a firm grasp on the concept of electronegativity itself. Electronegativity is a relative measure, meaning it's not an absolute property but rather a comparison of an atom's electron-attracting power relative to other atoms. Several scales exist to quantify electronegativity, with the most commonly used being the Pauling scale. On this scale, fluorine (F) is assigned the highest electronegativity value of 4.0, and other elements are ranked relative to fluorine.
Several factors influence an atom's electronegativity:
- Nuclear Charge: A higher nuclear charge (more protons) exerts a stronger pull on electrons.
- Atomic Radius: Smaller atoms have electrons closer to the nucleus, leading to a stronger attraction.
- Shielding Effect: Inner electrons shield outer electrons from the full nuclear charge, reducing the effective nuclear charge experienced by the outer electrons.
Comparing the Electronegativity of Carbon (C) and Nitrogen (N)
According to the Pauling scale, nitrogen (N) has a higher electronegativity than carbon (C). Nitrogen's electronegativity is approximately 3.0, while carbon's is approximately 2.5. This difference, though seemingly small, has significant consequences in chemical bonding and molecular properties.
Why is Nitrogen More Electronegative than Carbon?
The difference in electronegativity between nitrogen and carbon stems from the interplay of the factors mentioned earlier:
- Nuclear Charge: Nitrogen (atomic number 7) has a higher nuclear charge than carbon (atomic number 6). This means nitrogen's nucleus exerts a stronger pull on its electrons compared to carbon's nucleus.
- Atomic Radius: Nitrogen has a slightly smaller atomic radius than carbon. The closer proximity of the valence electrons to the nucleus in nitrogen enhances the attractive force.
- Shielding Effect: While both nitrogen and carbon have electrons in inner shells that shield the valence electrons, the effect is slightly less pronounced in nitrogen due to its smaller size.
The combined effect of a higher nuclear charge and a smaller atomic radius results in a stronger attraction of electrons to the nitrogen nucleus, making nitrogen more electronegative than carbon. The difference, though relatively small on the Pauling scale, is enough to significantly impact the properties of molecules containing both carbon and nitrogen.
Implications of the Electronegativity Difference
The fact that nitrogen is more electronegative than carbon has several important implications in chemistry, influencing various aspects of molecular behavior:
1. Polarity of Bonds
When carbon and nitrogen form a covalent bond (as in many organic compounds containing amines, amides, nitriles, etc.), the bond is polar. The nitrogen atom, being more electronegative, attracts the shared electron pair more strongly, creating a partial negative charge (δ-) on the nitrogen and a partial positive charge (δ+) on the carbon. This polarity influences the molecule's overall dipole moment and its interactions with other molecules.
2. Reactivity
The difference in electronegativity dictates the reactivity of molecules. The polar C-N bond is more susceptible to nucleophilic attacks because the carbon atom carries a partial positive charge, making it a good electrophile. Conversely, the nitrogen atom, with its partial negative charge, can act as a nucleophile. This difference in reactivity is fundamental in many organic reactions, including the synthesis of numerous biologically important molecules.
3. Hydrogen Bonding
Nitrogen's higher electronegativity plays a crucial role in hydrogen bonding. Nitrogen, especially when bonded to hydrogen (as in amines and amides), can act as a hydrogen bond acceptor and donor. This ability significantly impacts the physical properties of molecules containing N-H bonds, such as their boiling points and solubility in polar solvents. Hydrogen bonding is essential for the stability and function of proteins and nucleic acids.
4. Acid-Base Properties
The electronegativity difference between carbon and nitrogen influences the acid-base properties of molecules containing both elements. For instance, amines are weak bases because the nitrogen atom can accept a proton (H+), becoming positively charged. The electronegativity of nitrogen stabilizes this positive charge, making the amine a weaker base than it would be otherwise.
5. Spectroscopy
The electronegativity difference between carbon and nitrogen affects the chemical shifts observed in nuclear magnetic resonance (NMR) spectroscopy. The more electronegative nitrogen atom deshields the adjacent carbon nuclei, resulting in a downfield shift in the carbon NMR spectrum. This effect allows chemists to distinguish carbon atoms bonded to nitrogen from those bonded to other atoms.
Examples in Organic Chemistry
Let's examine some specific examples to illustrate the impact of the electronegativity difference between carbon and nitrogen:
1. Nitriles (R-CN)
In nitriles, the carbon atom is bonded to a nitrogen atom via a triple bond. Due to nitrogen's higher electronegativity, the triple bond is polar, with the nitrogen atom carrying a partial negative charge. This polarity affects the nitrile's reactivity, making it susceptible to nucleophilic attack at the carbon atom.
2. Amines (R-NH2)
Amines contain a nitrogen atom bonded to one or more carbon atoms and two hydrogen atoms. The nitrogen's electronegativity makes the N-H bond polar, and the lone pair of electrons on nitrogen allows it to act as a base, accepting protons. The polar N-H bonds are also capable of hydrogen bonding, influencing the amines' physical properties.
3. Amides (R-CONH2)
Amides contain a carbonyl group (C=O) bonded to a nitrogen atom. The combination of the electronegative oxygen and nitrogen atoms leads to a highly polar molecule with substantial hydrogen bonding capabilities. This polarity and hydrogen bonding affect the amides' solubility and melting points. The amide bond is crucial in the structure of proteins, where it links amino acids.
Conclusion
In conclusion, nitrogen is demonstrably more electronegative than carbon. This seemingly small difference in electronegativity has profound effects on the chemical behavior of molecules containing both elements. The polarity of C-N bonds, the reactivity of molecules, hydrogen bonding capabilities, acid-base properties, and spectroscopic characteristics are all significantly influenced by this difference. Understanding the electronegativity of atoms and how it affects molecular properties is essential for predicting and explaining the vast array of chemical reactions and behaviors observed in organic and inorganic chemistry. This principle provides a foundational understanding for advanced chemical concepts and is crucial for anyone pursuing studies in chemistry or related fields.
Latest Posts
Latest Posts
-
What Is The Monomer Of Polypeptide
Mar 29, 2025
-
How Many Unpaired Electrons Does Manganese Have
Mar 29, 2025
-
Water Is Made Up Of Which Two Elements
Mar 29, 2025
-
Benzoic Acid And Sodium Bicarbonate Reaction
Mar 29, 2025
-
During Which Process Is Molecular Oxygen Produced In Photosynthesis
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Is C Or N More Electronegative . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
