Water Is Made Up Of Which Two Elements
News Leon
Mar 29, 2025 · 6 min read
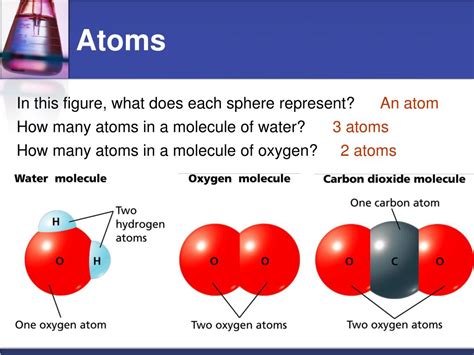
Table of Contents
Water: The Remarkable Compound Made of Two Simple Elements
Water. It's the lifeblood of our planet, essential for all known forms of life, and yet so deceptively simple in its composition. But what exactly is water, at its most fundamental level? The answer lies in its two constituent elements: hydrogen and oxygen. This seemingly straightforward answer opens the door to a fascinating exploration of chemistry, biology, and the very essence of life itself.
The Building Blocks: Hydrogen and Oxygen
Let's delve into the individual characteristics of these elements before understanding how their union creates the magic of water.
Hydrogen: The Lightest Element
Hydrogen (H), with its atomic number of 1, is the lightest element in the periodic table. Its single proton and single electron define its incredibly reactive nature. Hydrogen exists primarily as a diatomic molecule (H₂), meaning two hydrogen atoms share a covalent bond, a strong link formed by sharing electrons. This shared electron pair creates a relatively stable molecule, although it's still highly reactive under certain conditions, readily forming bonds with other elements, including oxygen. Its abundance in the universe is immense, making it a crucial component in countless chemical processes.
Oxygen: The Breath of Life
Oxygen (O), with its atomic number of 8, is a highly reactive nonmetal that plays a vital role in sustaining life on Earth. Like hydrogen, it typically exists as a diatomic molecule (O₂), crucial for respiration in most living organisms. The oxygen molecule's double bond, involving the sharing of four electrons, creates a relatively strong and stable molecule. However, its reactivity is responsible for its essential role in combustion and many other chemical reactions, including the vital process of cellular respiration. Oxygen's strong electronegativity – its tendency to attract electrons – will become crucial when we understand the water molecule.
The Formation of Water: A Covalent Bond
The magic of water begins with the chemical reaction between hydrogen and oxygen. Specifically, two hydrogen atoms bond with a single oxygen atom to form a molecule of water (H₂O). This bond is a covalent bond, characterized by the sharing of electrons between atoms. However, it's not an equal sharing.
Polarity: The Unequal Sharing of Electrons
Oxygen is significantly more electronegative than hydrogen. This means that the shared electrons in the covalent bond are more strongly attracted to the oxygen atom than to the hydrogen atoms. This unequal sharing of electrons results in a polar molecule, where one end of the molecule (the oxygen side) carries a slightly negative charge (δ-), and the other end (the hydrogen side) carries a slightly positive charge (δ+). This slight charge separation is what gives water its unique properties.
The Unique Properties of Water: A Consequence of its Structure
The seemingly simple H₂O molecule exhibits remarkable properties, many of which are directly attributable to its polarity and hydrogen bonding. These properties are essential for supporting life on Earth and for countless industrial and technological applications.
1. High Specific Heat Capacity: Temperature Regulation
Water has an exceptionally high specific heat capacity. This means it takes a significant amount of energy to raise the temperature of water. Conversely, water releases a large amount of energy as it cools. This property is crucial for regulating temperature, both on a global scale and within living organisms. Oceans, for instance, act as massive heat reservoirs, moderating climate fluctuations. Similarly, water within our bodies helps maintain a stable internal temperature.
2. High Heat of Vaporization: Cooling Mechanism
Water also has a high heat of vaporization. This means it requires a considerable amount of energy to convert liquid water into water vapor (evaporation). This property is vital for evaporative cooling. Sweating, for example, relies on the evaporative cooling effect of water to regulate body temperature. The energy required to evaporate the sweat is drawn from the body, leading to a cooling effect.
3. Excellent Solvent: The Universal Solvent
Water's polarity makes it an excellent solvent for many ionic and polar substances. The slightly positive hydrogen ends of water molecules attract negatively charged ions or polar molecules, while the slightly negative oxygen ends attract positively charged ions or polar molecules. This attraction allows water to dissolve many substances, earning it the title of "universal solvent." This property is essential for transporting nutrients and waste products in living organisms and for countless chemical reactions.
4. Cohesion and Adhesion: Surface Tension and Capillary Action
Water molecules exhibit strong cohesion (attraction to other water molecules) and adhesion (attraction to other substances). Cohesion is responsible for water's high surface tension, allowing insects to walk on water. Adhesion, combined with cohesion, contributes to capillary action, the ability of water to move against gravity in narrow tubes, a critical process in plant water transport.
5. Density Anomaly: Ice Floats
Water exhibits a unique density anomaly. Most substances become denser as they cool and solidify. However, ice (solid water) is less dense than liquid water, which is why ice floats. This property is crucial for aquatic life, as a layer of ice on the surface of a body of water insulates the water below, preventing it from freezing solid and allowing aquatic organisms to survive.
Water's Role in Life: From Cells to Ecosystems
The remarkable properties of water are fundamental to the existence and function of all known life forms.
1. Cellular Processes: The Medium of Life
Water serves as the primary medium for countless biochemical reactions within cells. It acts as a solvent, transporting nutrients, removing waste products, and facilitating enzyme activity. Cellular structures, such as cell membranes, depend on water's properties for their stability and function.
2. Photosynthesis: Powering Life
Water plays a crucial role in photosynthesis, the process by which plants convert light energy into chemical energy. Water molecules are split during photosynthesis, providing electrons to drive the energy-producing reactions. Oxygen, a byproduct of this process, is released into the atmosphere.
3. Respiration: Energy Release
Water is also a byproduct of cellular respiration, the process by which cells release energy from food molecules. This process utilizes oxygen to break down glucose, releasing energy and producing water and carbon dioxide.
4. Ecosystem Function: Shaping the World
Water's properties are crucial for the structure and function of entire ecosystems. Its ability to dissolve and transport nutrients, regulate temperature, and provide a habitat for countless organisms makes it fundamental to the biodiversity of our planet. From vast oceans to tiny puddles, water shapes the landscape and supports the intricate web of life.
Beyond H₂O: Different Forms of Water
While H₂O represents the most common form of water, it’s important to acknowledge that water can exist in various forms:
- Ice: The solid state of water, characterized by a crystalline structure.
- Liquid Water: The most common state, exhibiting the remarkable properties discussed earlier.
- Water Vapor: The gaseous state, crucial for atmospheric processes and weather patterns.
- Heavy Water: A naturally occurring isotope of water where the hydrogen atoms are replaced by deuterium (²H), having a different atomic mass and slightly different properties.
Conclusion: The Simple Wonder of Water
The seemingly simple combination of two hydrogen atoms and one oxygen atom creates a molecule with astonishing properties, making water the cornerstone of life on Earth. Understanding the chemical structure of water and its unique characteristics provides a deeper appreciation for its fundamental role in everything from cellular processes to global climate regulation. The study of water continues to fascinate scientists, offering endless opportunities for discovery and a deeper understanding of the world around us. From its simple molecular structure to its complex interactions with life, water truly remains a wonder of nature.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of A Mixture
Mar 31, 2025
-
What Is The Reciprocal Of 1 6
Mar 31, 2025
-
What Mineral Is The Hardest Known Substance In Nature
Mar 31, 2025
-
Which Organelle Is Enclosed By A Double Membrane
Mar 31, 2025
-
Compare And Contrast An Ecosystem And A Habitat
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Water Is Made Up Of Which Two Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
