How Many Unpaired Electrons Does Manganese Have
News Leon
Mar 29, 2025 · 5 min read
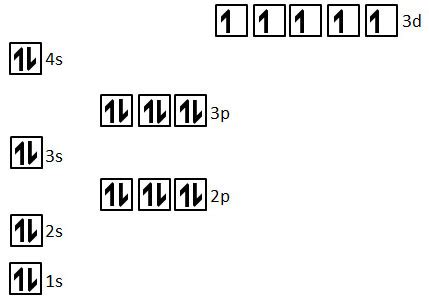
Table of Contents
How Many Unpaired Electrons Does Manganese Have? A Deep Dive into Electronic Configuration and Magnetic Properties
Manganese (Mn), a transition metal residing in the 7th group of the periodic table, boasts a fascinating electronic structure that significantly impacts its chemical behavior and magnetic properties. Understanding its electron configuration is key to determining the number of unpaired electrons it possesses. This article will delve into the intricacies of manganese's electronic structure, exploring its implications for various applications and properties.
Unveiling Manganese's Electronic Configuration
To ascertain the number of unpaired electrons in manganese, we must first understand its electronic configuration. The atomic number of manganese is 25, meaning it has 25 electrons. Following the Aufbau principle and Hund's rule, these electrons fill the orbitals in a specific order, minimizing the total energy of the atom.
The electronic configuration of a neutral manganese atom is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵.
Let's break this down:
-
1s², 2s², 2p⁶, 3s², 3p⁶: These represent the filled inner shells, with all electrons paired. These electrons are relatively tightly bound to the nucleus and don't participate significantly in chemical bonding or magnetic behavior.
-
4s²: The 4s subshell is filled with two paired electrons.
-
3d⁵: This is where things get interesting. The 3d subshell has five orbitals, each capable of holding two electrons. According to Hund's rule, electrons will individually occupy each orbital before pairing up. Therefore, in the 3d⁵ configuration of manganese, each of the five 3d orbitals contains one unpaired electron.
The Significance of Unpaired Electrons
The presence of unpaired electrons is directly responsible for many of manganese's notable properties, including:
-
Paramagnetism: Substances with unpaired electrons are paramagnetic, meaning they are weakly attracted to an external magnetic field. The unpaired electrons' magnetic moments align with the external field, resulting in a net magnetic attraction. Manganese's five unpaired electrons contribute significantly to its paramagnetic character.
-
Variable Oxidation States: The presence of unpaired electrons in the d-orbital allows manganese to readily lose electrons and exhibit multiple oxidation states. This flexibility is crucial for its role in various chemical reactions and its diverse applications. Common oxidation states include +2, +3, +4, +6, and +7, each with a different number of unpaired electrons depending on the specific electronic configuration of the Mn ion.
-
Catalytic Activity: Manganese's ability to easily gain or lose electrons, coupled with its unpaired electrons, makes it an excellent catalyst in various chemical processes. It can facilitate electron transfer between reactants, speeding up reaction rates.
-
Color in Compounds: The d-orbital electrons, especially the unpaired ones, are responsible for the absorption and emission of light in transition metal compounds. This results in the characteristic colors observed in many manganese-containing compounds. The specific color depends on the oxidation state and ligand environment around the manganese ion.
Manganese in Different Oxidation States: A Closer Look
The number of unpaired electrons in manganese varies depending on its oxidation state. Let's consider some key oxidation states:
-
Mn²⁺ (Manganese(II)): In this state, manganese loses two electrons, both from the 4s orbital. The 3d orbitals retain their five unpaired electrons. Therefore, Mn²⁺ has five unpaired electrons.
-
Mn³⁺ (Manganese(III)): Losing one more electron typically occurs from a 3d orbital. This leaves four unpaired electrons in Mn³⁺. However, depending on the ligand field strength, the arrangement of electrons can slightly change, affecting the exact number of unpaired electrons.
-
Mn⁴⁺ (Manganese(IV)): Further electron loss results in three unpaired electrons. Again, ligand field effects can influence the exact electron configuration and the number of unpaired electrons.
-
Mn⁷⁺ (Manganese(VII)): In its highest oxidation state (+7), as seen in permanganate ion (MnO₄⁻), manganese has lost all its 4s and 3d electrons. Thus, Mn⁷⁺ has zero unpaired electrons. This explains the lack of paramagnetism in permanganate.
Impact on Magnetic Properties
The number of unpaired electrons directly influences the magnetic properties of manganese and its compounds. The paramagnetism mentioned earlier is directly proportional to the number of unpaired electrons. Higher numbers of unpaired electrons lead to stronger paramagnetic behavior.
However, the interaction between the unpaired electrons and the surrounding ligands (atoms or ions bonded to the manganese ion) also plays a crucial role. The ligand field strength can affect the energy levels of the d-orbitals, sometimes leading to pairing of electrons and a reduction in paramagnetism or even the emergence of ferromagnetism or antiferromagnetism in certain manganese compounds.
Applications Leveraging Manganese's Unique Properties
The unique combination of variable oxidation states and magnetic properties derived from its unpaired electrons makes manganese an essential element in numerous applications:
-
Alloys: Manganese is added to steel alloys to improve their strength, hardness, and toughness. Its presence modifies the microstructure and enhances the overall mechanical properties of the alloy.
-
Pigments: Manganese compounds are used as pigments in paints, plastics, and ceramics. The color varies based on the oxidation state and the specific compound.
-
Batteries: Manganese dioxide (MnO₂) is a key component in many dry-cell batteries, serving as the cathode material.
-
Fertilizers: Manganese is an essential micronutrient for plants, playing a vital role in photosynthesis and other metabolic processes. Manganese-containing fertilizers provide this crucial element to crops.
-
Catalysis: Manganese-based catalysts find applications in various industrial processes, including the production of chemicals, plastics, and fuels. They facilitate crucial reactions due to manganese's ability to readily accept and donate electrons.
Conclusion: Unpaired Electrons – The Heart of Manganese's Functionality
The number of unpaired electrons in manganese, typically five in its neutral state, is the cornerstone of its versatile chemical behavior and diverse applications. Understanding its electronic configuration and the impact of unpaired electrons on its properties allows us to appreciate its importance in numerous fields, from materials science to biology. The influence of oxidation state and ligand field effects further expands the spectrum of possibilities, highlighting the complex and intriguing nature of this transition metal. This deep dive into manganese's electronic structure provides a solid foundation for further exploration of its fascinating chemistry and its significant role in the modern world. The interaction between unpaired electrons, oxidation states, and ligand fields contributes to a rich tapestry of magnetic and chemical properties, making manganese a truly remarkable element.
Latest Posts
Latest Posts
-
Oxidation Number Of S In So2
Mar 31, 2025
-
Reduction Of An Aldehyde Produces A
Mar 31, 2025
-
Which Of The Following Is An Example Of A Mixture
Mar 31, 2025
-
What Is The Reciprocal Of 1 6
Mar 31, 2025
-
What Mineral Is The Hardest Known Substance In Nature
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Manganese Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
