How Many Valence Electrons In Cl
News Leon
Mar 25, 2025 · 5 min read
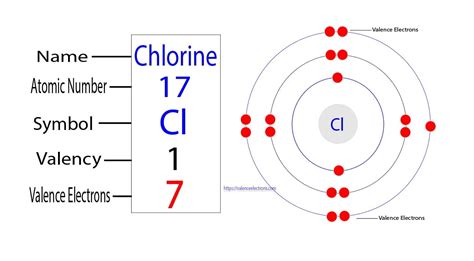
Table of Contents
How Many Valence Electrons Does Chlorine (Cl) Have? A Deep Dive into Atomic Structure and Chemical Behavior
Chlorine (Cl), a vibrant yellowish-green gas, plays a crucial role in various aspects of our lives, from purifying water to forming essential compounds in our bodies. Understanding its chemical behavior hinges on knowing its valence electrons. This article delves deep into the answer to the question: how many valence electrons does chlorine have? We'll explore the underlying atomic structure, its implications for bonding, and further solidify your understanding of this fundamental concept in chemistry.
Understanding Valence Electrons: The Key to Chemical Reactivity
Before focusing specifically on chlorine, let's establish a clear understanding of valence electrons. These are the electrons located in the outermost shell (also known as the valence shell) of an atom. They are the key players in chemical reactions, determining how an atom will interact with other atoms to form molecules and compounds. The number of valence electrons dictates an element's reactivity and the types of bonds it can form.
Atoms strive for stability, often achieving this by having a full valence shell. This usually means eight electrons (the octet rule), although there are exceptions, particularly with elements having lower atomic numbers. Atoms will gain, lose, or share electrons to reach this stable configuration. This process is the foundation of chemical bonding.
Determining Valence Electrons: Electronic Configuration and the Periodic Table
The number of valence electrons an atom possesses can be determined in two primary ways:
1. Electronic Configuration: A Look Inside the Atom
The electronic configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. For chlorine (Cl), which has an atomic number of 17, its electronic configuration is 1s²2s²2p⁶3s²3p⁵. This notation tells us:
- 1s²: Two electrons in the first energy level (n=1), in the s subshell.
- 2s²: Two electrons in the second energy level (n=2), in the s subshell.
- 2p⁶: Six electrons in the second energy level (n=2), in the p subshell.
- 3s²: Two electrons in the third energy level (n=3), in the s subshell.
- 3p⁵: Five electrons in the third energy level (n=3), in the p subshell.
The outermost shell is the third energy level (n=3). Adding the electrons in this shell (3s² + 3p⁵) gives us a total of seven valence electrons for chlorine.
2. The Periodic Table: A Quick and Easy Method
The periodic table is a powerful tool that organizes elements based on their atomic structure and properties. The group number (vertical column) of an element in the periodic table often directly indicates the number of valence electrons. Chlorine belongs to Group 17 (or VIIA), also known as the halogens. Group 17 elements generally have seven valence electrons.
Therefore, by simply knowing chlorine's position in the periodic table, we can quickly deduce it has seven valence electrons.
Chlorine's Chemical Behavior: The Impact of Seven Valence Electrons
Having seven valence electrons profoundly influences chlorine's chemical behavior. It's one electron short of a stable octet, making it highly reactive. To achieve a stable electron configuration, chlorine readily:
-
Gains one electron: This forms a chloride ion (Cl⁻), which has a full octet of eight electrons. This is a common process, particularly in ionic bonding with metals. For example, in sodium chloride (NaCl), sodium (Na) donates an electron to chlorine, forming Na⁺ and Cl⁻ ions held together by electrostatic attraction.
-
Shares one electron: This process leads to covalent bonding, where chlorine shares an electron pair with another atom, usually a non-metal. Examples include hydrogen chloride (HCl) and chlorine gas (Cl₂). In Cl₂, two chlorine atoms share one electron pair to achieve a stable octet.
Applications of Chlorine and its Compounds: From Water Purification to Medicine
The chemical reactivity of chlorine, dictated by its seven valence electrons, underpins its widespread applications:
1. Water Purification: A Lifesaver
Chlorine's powerful disinfectant properties are widely used in water treatment plants. It effectively kills harmful bacteria, viruses, and other microorganisms, making water safe for drinking and other uses. The effectiveness stems from its ability to react with organic matter and disrupt cellular processes in pathogens.
2. Industrial Processes: Versatile Uses
Chlorine finds various applications in industrial settings. It's a crucial component in the production of various chemicals, including plastics (PVC), solvents, and pesticides. Its reactivity allows it to participate in many synthesis processes.
3. Medical Applications: Essential for Health
Chlorine compounds play vital roles in medicine. For instance, sodium hypochlorite (NaClO), the active ingredient in bleach, is a potent disinfectant used in healthcare settings. Other chlorine-containing compounds find applications as antiseptics and pharmaceuticals.
4. Other Everyday Applications
Beyond these major applications, chlorine is present in various everyday products, including:
- Food processing: Certain chlorine-containing compounds are used as preservatives and disinfectants in food processing.
- Textiles: Chlorine-based bleaches are used in the textile industry to whiten fabrics.
- Paper production: Chlorine is involved in bleaching processes during paper manufacturing.
Beyond the Octet Rule: Exceptions and Nuances
While the octet rule is a useful guideline, it's not absolute. Some molecules and compounds have exceptions. For instance, certain molecules with atoms having less than eight valence electrons exist, and these are often referred to as electron-deficient molecules. Understanding these exceptions requires a deeper dive into advanced bonding theories and molecular orbital theory, but they don't invalidate the fundamental principle that the number of valence electrons determines chemical reactivity.
Conclusion: Valence Electrons – The Foundation of Chlorine's Chemistry
In summary, chlorine (Cl) possesses seven valence electrons. This fundamental property dictates its high reactivity, its ability to form both ionic and covalent bonds, and its wide-ranging applications across various industries and everyday life. Understanding the concept of valence electrons and how it applies to chlorine provides a solid foundation for comprehending its chemical behavior and the significance of this element in our world. By applying this knowledge and exploring other aspects of its chemical properties, we can better appreciate the crucial role chlorine plays in various fields, from ensuring safe drinking water to contributing to advancements in medicine and industry. The seemingly simple question, "How many valence electrons does chlorine have?" leads to a deep and fascinating exploration of atomic structure and chemical bonding.
Latest Posts
Latest Posts
-
How Many Electrons Are In C
Mar 29, 2025
-
Reaction Of Ethyl Alcohol With Acetic Acid
Mar 29, 2025
-
What Disappears The Moment You Say It
Mar 29, 2025
-
Microscopic Study Of Tissues Is Called
Mar 29, 2025
-
What Are Characteristics Of A Base
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Cl . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
