Reaction Of Ethyl Alcohol With Acetic Acid
News Leon
Mar 29, 2025 · 5 min read
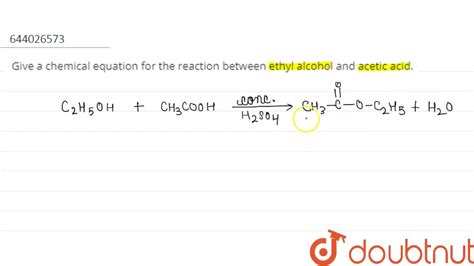
Table of Contents
The Esterification Reaction: A Deep Dive into Ethyl Alcohol and Acetic Acid
The reaction between ethyl alcohol (ethanol) and acetic acid is a classic example of an esterification reaction, a fundamental process in organic chemistry with widespread applications in various industries. This reaction, producing ethyl acetate, a fragrant ester commonly used as a solvent, offers a rich learning opportunity to explore reaction mechanisms, kinetics, and equilibrium. This comprehensive article will delve into the intricacies of this reaction, exploring its mechanism, factors influencing its rate and yield, and its significant industrial applications.
Understanding the Reactants: Ethyl Alcohol and Acetic Acid
Before diving into the reaction itself, let's establish a firm understanding of the two key reactants: ethyl alcohol and acetic acid.
Ethyl Alcohol (Ethanol): The Alcohol Reactant
Ethanol, also known as ethyl alcohol, is a simple alcohol with the chemical formula CH₃CH₂OH. It's a colorless, volatile, and flammable liquid with a characteristic pleasant odor. Ethanol is widely known for its presence in alcoholic beverages, but it also has significant industrial uses as a solvent, fuel, and precursor for the synthesis of various chemicals, including ethyl acetate. Its hydroxyl (-OH) group is crucial for its reactivity in esterification.
Acetic Acid: The Carboxylic Acid Reactant
Acetic acid, with the chemical formula CH₃COOH, is a weak organic acid that is the main component of vinegar. It's a colorless liquid with a pungent, vinegary odor. The carboxyl group (-COOH), characterized by a carbonyl group (C=O) adjacent to a hydroxyl group (-OH), is the key functional group responsible for its acidic properties and its participation in esterification reactions. The acidic hydrogen of the carboxyl group is readily available for reaction.
The Esterification Reaction: Mechanism and Kinetics
The reaction between ethyl alcohol and acetic acid is a reversible reaction, meaning it proceeds in both the forward and reverse directions. The forward reaction produces ethyl acetate and water, while the reverse reaction (hydrolysis) breaks down ethyl acetate into ethanol and acetic acid.
The Reaction Equation:
The overall reaction can be represented by the following equation:
CH₃COOH + CH₃CH₂OH ⇌ CH₃COOCH₂CH₃ + H₂O
Acetic acid + Ethanol ⇌ Ethyl acetate + Water
The Detailed Mechanism:
The esterification reaction is typically acid-catalyzed, meaning it proceeds faster in the presence of an acid catalyst, usually a strong mineral acid like sulfuric acid or hydrochloric acid. The mechanism involves several steps:
-
Protonation of the carbonyl oxygen: The acid catalyst protonates the carbonyl oxygen of the acetic acid, making the carbonyl carbon more electrophilic (electron-deficient).
-
Nucleophilic attack by ethanol: The oxygen atom of the ethanol molecule, acting as a nucleophile (electron-rich species), attacks the electrophilic carbonyl carbon of the protonated acetic acid.
-
Proton transfer: A proton is transferred from the hydroxyl group of the newly formed intermediate to the oxygen atom of the ethoxy group.
-
Elimination of water: A molecule of water is eliminated from the intermediate, forming the ester, ethyl acetate.
-
Deprotonation: The proton from the acid catalyst is removed, regenerating the catalyst.
Factors Affecting the Rate and Yield:
Several factors significantly influence the rate and yield of the esterification reaction:
-
Temperature: Increasing the temperature generally increases the reaction rate, but high temperatures might lead to side reactions.
-
Concentration of reactants: Higher concentrations of both reactants usually lead to a faster reaction rate and higher yield, according to the law of mass action.
-
Acid catalyst concentration: The presence of an acid catalyst accelerates the reaction, but excessive catalyst concentration might not proportionally increase the yield.
-
Water removal: Since the reaction is reversible, removing the water produced shifts the equilibrium to the right, favoring ester formation and improving the yield. This can be achieved through techniques like azeotropic distillation.
-
Reaction time: Sufficient reaction time is crucial to allow the reaction to reach equilibrium and achieve a satisfactory yield.
Industrial Applications of Ethyl Acetate
Ethyl acetate, the product of the reaction between ethyl alcohol and acetic acid, finds extensive use in various industries:
-
Solvent: Ethyl acetate is a widely used solvent in the production of paints, varnishes, lacquers, and adhesives. Its volatility and ability to dissolve various substances make it ideal for these applications.
-
Extraction: In the food and pharmaceutical industries, ethyl acetate is employed as a solvent for extracting flavors, fragrances, and other valuable compounds.
-
Cleaning agent: Its effectiveness in dissolving fats and oils makes it a component in certain cleaning agents.
-
Nail polish remover: Ethyl acetate is a common ingredient in nail polish remover due to its ability to dissolve the common polymers in nail polishes.
-
Chemical intermediate: Ethyl acetate serves as a crucial intermediate in the synthesis of other chemicals and pharmaceuticals.
Equilibrium and Le Chatelier's Principle
The esterification reaction is an equilibrium process. According to Le Chatelier's principle, any change in the reaction conditions will shift the equilibrium position to counteract the change. As mentioned earlier, removing water from the reaction mixture drives the equilibrium toward the product side, increasing the yield of ethyl acetate. Conversely, adding water would favor the reverse reaction, hydrolysis, resulting in a decrease in ethyl acetate and an increase in acetic acid and ethanol.
Safety Precautions
When performing esterification reactions, especially those involving concentrated acids, it's crucial to follow proper safety precautions. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. The reaction should be carried out in a well-ventilated area or under a fume hood to minimize exposure to volatile organic compounds. Proper waste disposal methods should be followed to handle the acidic waste products safely.
Conclusion: A Versatile Reaction with Broad Applications
The reaction between ethyl alcohol and acetic acid, leading to the formation of ethyl acetate, is a quintessential example of an esterification reaction, highlighting the beauty and elegance of organic chemistry. Understanding the reaction mechanism, kinetics, and equilibrium is essential for optimizing the reaction conditions to achieve high yields of ethyl acetate. The widespread industrial applications of ethyl acetate further underscore the significance of this seemingly simple reaction. This detailed exploration emphasizes the importance of mastering this reaction for anyone aspiring to work in chemistry or related fields. The principles discussed here can also be applied to understanding other esterification reactions, showcasing their versatility and importance in organic synthesis.
Latest Posts
Latest Posts
-
Is Sodium Methoxide A Strong Nucleophile
Mar 31, 2025
-
The Amount Of Space An Object Occupies
Mar 31, 2025
-
A Small Object Begins A Free Fall From A Height
Mar 31, 2025
-
Which Strand Of Dna Serves As The Template For Transcription
Mar 31, 2025
-
Is Alcl3 An Acid Or Base
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Ethyl Alcohol With Acetic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
