What Are Characteristics Of A Base
News Leon
Mar 29, 2025 · 6 min read
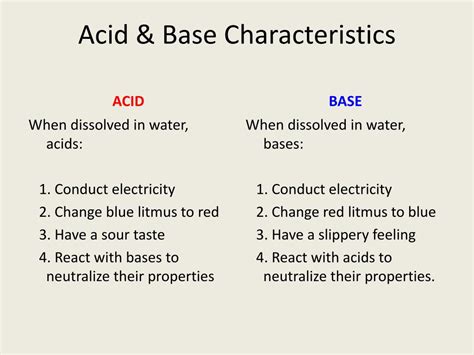
Table of Contents
What are the Characteristics of a Base?
Understanding the characteristics of bases is fundamental to chemistry and many related fields. Bases, along with acids, form the cornerstone of acid-base chemistry, a vital concept in everything from industrial processes to biological systems. This comprehensive guide will delve into the defining characteristics of bases, exploring their properties, reactions, and applications.
Defining Bases: More Than Just a Bitter Taste
While the popular understanding might associate bases with a bitter taste and slippery feel (like soap), a more scientific definition is crucial. A base, in its simplest form, is a substance that can accept a proton (H⁺) or donate a pair of electrons. This dual definition encompasses the various ways bases interact in chemical reactions. We'll explore both aspects in detail.
1. Proton Acceptors (Brønsted-Lowry Definition):
The Brønsted-Lowry theory defines a base as a proton acceptor. This means a base reacts with an acid by accepting a proton from the acid. This reaction results in the formation of a conjugate acid and a conjugate base. Consider the classic reaction between hydrochloric acid (HCl) and water (H₂O):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
In this reaction, water acts as a base because it accepts a proton from hydrochloric acid. Hydrochloric acid donates the proton, acting as an acid. The resulting hydronium ion (H₃O⁺) is the conjugate acid of water, and the chloride ion (Cl⁻) is the conjugate base of hydrochloric acid. This exemplifies the fundamental principle of acid-base reactions as a proton transfer process.
2. Electron Pair Donors (Lewis Definition):
The Lewis definition broadens the scope of bases even further. A Lewis base is defined as an electron pair donor. This definition encompasses substances that might not strictly accept a proton but can donate a lone pair of electrons to form a coordinate covalent bond with an electron-deficient species (a Lewis acid). For example, ammonia (NH₃) acts as a Lewis base when it reacts with boron trifluoride (BF₃):
NH₃ + BF₃ → H₃N-BF₃
Here, ammonia donates a lone pair of electrons to boron trifluoride, which lacks an octet of electrons. The resulting adduct (H₃N-BF₃) is formed through a coordinate covalent bond. This definition highlights that basicity isn't solely about proton acceptance; it also involves the availability of electron pairs for bonding.
Key Characteristics of Bases: A Detailed Look
Beyond the fundamental definitions, several characteristics help identify and understand bases:
1. pH Greater Than 7:
In aqueous solutions, bases have a pH greater than 7. The pH scale measures the concentration of hydrogen ions (H⁺) in a solution. A high pH indicates a low concentration of H⁺ ions, which is characteristic of basic solutions. Strong bases have a pH closer to 14, while weak bases have a pH slightly above 7.
2. Bitter Taste (Caution!):
Many bases exhibit a bitter taste. However, it is crucial to never taste an unknown substance to determine its properties. Many bases are corrosive and can cause severe damage to the mouth and throat. This characteristic should only be mentioned for illustrative purposes, not for experimental verification.
3. Slippery Feel:
Many bases have a slippery or soapy feel. This is due to the reaction of bases with the oils and fats on the skin, producing soap-like substances. Again, caution should be exercised when handling bases due to potential skin irritation and damage.
4. Reaction with Acids: Neutralization:
Bases react with acids in a neutralization reaction. This reaction involves the combination of H⁺ ions from the acid and OH⁻ ions from the base to form water (H₂O). The other product is a salt, which is an ionic compound formed from the cation of the base and the anion of the acid. For example, the reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) produces sodium chloride (NaCl) and water:
NaOH + HCl → NaCl + H₂O
This neutralization reaction is exothermic, meaning it releases heat.
5. Conductivity of Electricity:
Many bases, particularly strong bases, are good conductors of electricity in aqueous solutions. This is because they dissociate into ions (cations and anions) in water, which can carry an electric current. The greater the degree of dissociation, the higher the conductivity.
6. Indicators Change Color:
Certain indicators change color in the presence of bases. These indicators are typically weak acids or bases that exhibit different colors in their acidic and basic forms. Litmus paper, for example, turns blue in basic solutions, providing a visual indication of basicity. Phenolphthalein is another common indicator that turns pink in basic solutions.
Types of Bases: Strength and Solubility
Bases are classified into several categories based on their strength and solubility:
1. Strong Bases:
Strong bases completely dissociate into their constituent ions in water. They have a high concentration of hydroxide ions (OH⁻) in solution. Examples include:
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
- Calcium hydroxide (Ca(OH)₂)
2. Weak Bases:
Weak bases only partially dissociate in water. They have a lower concentration of hydroxide ions compared to strong bases. Examples include:
- Ammonia (NH₃)
- Pyridine (C₅H₅N)
- Many organic amines
3. Soluble and Insoluble Bases:
Bases can also be classified based on their solubility in water. Soluble bases readily dissolve in water, while insoluble bases do not. The solubility of a base is determined by the nature of its cation and anion.
Applications of Bases: A Wide Range of Uses
Bases have a wide array of applications in various fields:
1. Industrial Applications:
- Chemical manufacturing: Bases are used extensively in the production of various chemicals, including fertilizers, detergents, and plastics.
- Pulp and paper industry: Bases are used in the process of breaking down wood fibers to produce paper pulp.
- Metal refining: Bases are used in the purification and extraction of metals.
- Water treatment: Bases are used to neutralize acidic water and adjust its pH.
2. Everyday Applications:
- Cleaning products: Many household cleaning products contain bases, which help remove grease and dirt.
- Soaps and detergents: Soaps are made by reacting fats and oils with bases.
- Baking: Some baking recipes use bases like baking soda (sodium bicarbonate) to help the batter rise.
- Agriculture: Bases are sometimes used to adjust the pH of soil for optimal plant growth.
3. Biological Applications:
- Maintaining pH balance: Many biological systems rely on buffers, which are solutions that resist changes in pH. These buffers often involve weak acids and their conjugate bases.
- Enzyme function: The activity of many enzymes depends on the pH of their environment. Bases are crucial in maintaining optimal pH for enzyme activity.
Conclusion: A Fundamental Concept
Understanding the characteristics of bases is essential for anyone studying chemistry or related fields. From their fundamental definitions as proton acceptors or electron pair donors to their diverse applications in various industries and biological systems, bases play a crucial role in our world. This guide has provided a comprehensive overview of the properties, reactions, and applications of bases, highlighting their importance in chemistry and beyond. Remember to always handle bases with care due to their potentially corrosive nature. Safe laboratory practices and appropriate personal protective equipment (PPE) should always be used when working with bases.
Latest Posts
Latest Posts
-
Compare And Contrast An Ecosystem And A Habitat
Mar 31, 2025
-
Network Layer Firewall Works As A
Mar 31, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Mar 31, 2025
-
The Amount Of Space An Object Occupies
Mar 31, 2025
-
A Small Object Begins A Free Fall From A Height
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Are Characteristics Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
