How Many Electrons Are In C
News Leon
Mar 29, 2025 · 6 min read
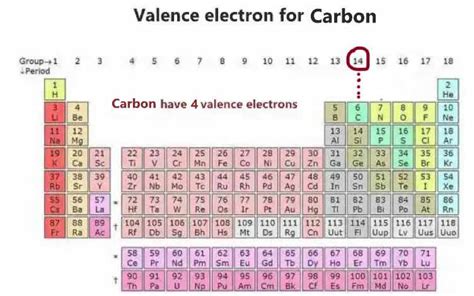
Table of Contents
How Many Electrons Are in C? Understanding Carbon's Electronic Structure
Carbon (C), the sixth element on the periodic table, is the fundamental building block of life as we know it. Its unique electronic structure is responsible for its remarkable versatility and ability to form an incredibly diverse range of molecules, from simple hydrocarbons to complex biomolecules like DNA. Understanding the number of electrons in a carbon atom is crucial to grasping its chemical behavior and the properties of the compounds it forms.
The Basics: Atomic Number and Electron Configuration
The atomic number of an element represents the number of protons in its nucleus. Since atoms are electrically neutral, the number of protons equals the number of electrons in a neutral atom. Carbon's atomic number is 6, meaning a neutral carbon atom possesses 6 electrons.
This seemingly simple fact unlocks a wealth of information about carbon's chemical reactivity. To understand this fully, we delve into the electron configuration, which describes how these electrons are arranged in various energy levels or shells surrounding the nucleus.
Electron Shells and Subshells: Unveiling Carbon's Structure
Electrons occupy specific energy levels, often visualized as concentric shells around the nucleus. These shells have a limited capacity for electrons. The first shell (n=1) can hold a maximum of two electrons, the second shell (n=2) can hold up to eight, and so on. Each shell is further divided into subshells, labeled s, p, d, and f, each with its own specific shape and capacity for electrons.
For carbon, the electron configuration is 1s²2s²2p². Let's break this down:
-
1s²: This indicates two electrons in the 1s subshell. The '1' represents the first energy level (shell), 's' represents the s subshell (which is spherical), and the '²' indicates that two electrons occupy this subshell. The s subshell can only hold a maximum of two electrons.
-
2s²: This denotes two electrons in the 2s subshell. The '2' represents the second energy level, 's' again represents the s subshell, and '²' shows two electrons filling this subshell.
-
2p²: This indicates two electrons in the 2p subshell. The '2' represents the second energy level, 'p' represents the p subshell (which has a dumbbell shape), and '²' indicates that two electrons occupy this subshell. The p subshell can hold a maximum of six electrons.
Therefore, a neutral carbon atom has:
- 2 electrons in the first shell (1s²)
- 4 electrons in the second shell (2s²2p²)
This arrangement is key to carbon's remarkable ability to form four covalent bonds, a concept explored further below.
Carbon's Valence Electrons: The Key to Bonding
The electrons in the outermost shell of an atom are called valence electrons. These are the electrons most involved in chemical bonding. For carbon, the valence electrons are the four electrons in the second shell (2s²2p²). These four valence electrons are responsible for carbon's tetravalency – its ability to form four covalent bonds with other atoms.
The concept of valence electrons is crucial in understanding chemical bonding and molecular structure. It explains why carbon can form a vast array of compounds with different elements and itself.
Covalent Bonding: How Carbon Shares Electrons
Carbon typically forms covalent bonds, where atoms share electrons to achieve a stable electron configuration, usually resembling that of a noble gas (a full outermost shell). Since carbon has four valence electrons, it needs to share four electrons to achieve a stable octet (eight electrons in its outermost shell).
This sharing of electrons leads to the formation of strong covalent bonds, explaining the strength and stability of many carbon-containing compounds. This is particularly prominent in organic chemistry, where carbon atoms bond extensively to form long chains, rings, and complex three-dimensional structures.
Carbon's Isotopes and Electron Numbers
While a neutral carbon atom always has six electrons, it's important to consider isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. The most common isotopes of carbon are carbon-12 (¹²C) and carbon-13 (¹³C), both with six protons and six electrons in their neutral state. Carbon-14 (¹⁴C), a radioactive isotope, also possesses six electrons in its neutral form. The number of neutrons only affects the mass of the atom, not the number of electrons.
Therefore, regardless of the isotope, a neutral carbon atom consistently contains six electrons.
Ions: When Carbon Gains or Loses Electrons
While neutral carbon atoms have six electrons, it's important to acknowledge the possibility of carbon forming ions. An ion is an atom or molecule with a net electrical charge due to the loss or gain of electrons. Carbon can theoretically form both positive (cations) and negative (anions) ions, although this is less common compared to its covalent bonding behavior.
-
Carbon Cations (C⁺, C²⁺, etc.): These are formed when carbon loses one or more electrons. The resulting ion would have fewer than six electrons.
-
Carbon Anions (C⁻, C²⁻, etc.): These are formed when carbon gains one or more electrons. The resulting ion would have more than six electrons.
However, these ionic forms are less stable and less frequently observed than carbon's covalently bonded compounds. Carbon's preference for covalent bonding reflects its position in the periodic table and its electronegativity.
Carbon's Role in Organic Chemistry: The Backbone of Life
The unique electronic structure of carbon is the foundation of organic chemistry, the study of carbon-containing compounds. Carbon's ability to form four strong covalent bonds with a variety of atoms, including other carbon atoms, allows for the formation of long chains, branched structures, rings, and complex three-dimensional molecules.
This ability is fundamental to the existence of life. Carbohydrates, lipids, proteins, and nucleic acids—the fundamental building blocks of life—are all based on carbon's ability to form diverse and complex structures. Understanding carbon's electronic structure is thus crucial for comprehending the chemistry of life itself.
Beyond Organic Chemistry: Carbon's Diverse Applications
Carbon's versatility extends far beyond organic chemistry. Various allotropes of carbon, such as diamond and graphite, exhibit vastly different properties due to the arrangement of their carbon atoms. Diamonds, with their strong covalent bonds in a tetrahedral arrangement, are renowned for their hardness and brilliance. Graphite, with its layered structure, is known for its softness and conductivity. Fullerenes, like buckminsterfullerene (C₆₀), represent another fascinating allotrope with unique properties and potential applications.
Carbon nanotubes, a newly discovered allotrope, hold immense promise due to their exceptional strength, electrical conductivity, and potential applications in nanotechnology. These materials demonstrate the wide range of properties achievable with variations in carbon's structure.
Conclusion: The Significance of Carbon's Six Electrons
The seemingly simple answer – a neutral carbon atom has six electrons – unlocks a vast understanding of carbon's fundamental chemistry and its crucial role in the universe. Its electronic configuration, with four valence electrons, explains its ability to form an immense diversity of compounds, from simple molecules to complex biomolecules. This characteristic makes carbon unique among elements and is the reason for its ubiquitous presence in both organic and inorganic chemistry, ultimately playing a central role in the very fabric of life. Understanding this core aspect is crucial for advancements across numerous scientific disciplines.
Latest Posts
Latest Posts
-
Which Organelle Is Enclosed By A Double Membrane
Mar 31, 2025
-
Compare And Contrast An Ecosystem And A Habitat
Mar 31, 2025
-
Network Layer Firewall Works As A
Mar 31, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Mar 31, 2025
-
The Amount Of Space An Object Occupies
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In C . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
