How Many Valence Electrons Are In Krypton
News Leon
Mar 27, 2025 · 4 min read
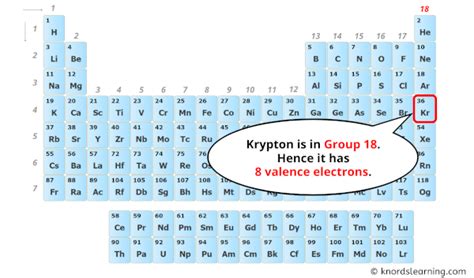
Table of Contents
How Many Valence Electrons Are in Krypton? A Deep Dive into Electron Configuration and Chemical Properties
Krypton, a noble gas with the symbol Kr and atomic number 36, holds a unique position in the periodic table. Its chemical inertness, largely attributed to its electron configuration, makes it a fascinating subject for studying atomic structure and chemical bonding. This article delves deep into the question: How many valence electrons are in krypton? We'll explore the electron configuration, its implications for reactivity, and its applications, providing a comprehensive understanding of this element.
Understanding Electron Configuration and Valence Electrons
Before we answer the central question, let's establish a foundational understanding of electron configuration and valence electrons.
Electron configuration describes the arrangement of electrons within an atom's electron shells and subshells. It follows specific rules based on quantum mechanics, with electrons occupying orbitals of increasing energy levels. This arrangement dictates an atom's chemical behavior.
Valence electrons are the electrons located in the outermost shell (valence shell) of an atom. These are the electrons that participate in chemical bonding, determining an atom's reactivity and the types of bonds it can form. The number of valence electrons is crucial for predicting the chemical properties of an element.
Determining Krypton's Valence Electrons
Krypton's atomic number is 36, indicating it has 36 protons and, in a neutral atom, 36 electrons. To determine its valence electrons, we need to understand its electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
This configuration shows how Krypton's 36 electrons are distributed among its shells and subshells.
- 1s²: Two electrons in the first shell (n=1).
- 2s² 2p⁶: Eight electrons in the second shell (n=2).
- 3s² 3p⁶: Eight electrons in the third shell (n=3).
- 4s² 3d¹⁰ 4p⁶: Eighteen electrons in the fourth shell (n=4).
The outermost shell for krypton is the fourth shell (n=4). This shell contains a total of eight electrons (4s² 4p⁶). Therefore, krypton has 8 valence electrons.
The Significance of Eight Valence Electrons
The presence of eight valence electrons in krypton is highly significant. This configuration is known as a stable octet. Atoms strive to achieve a stable octet, either by gaining, losing, or sharing electrons to fill their valence shell. Atoms with a complete octet are generally unreactive.
This explains krypton's inertness. It has no tendency to gain, lose, or share electrons because its valence shell is already full. This makes it a noble gas, a group of elements known for their extremely low reactivity.
Krypton's Chemical Properties and Inertness
Krypton's chemical inertness is a direct consequence of its filled valence shell. It rarely participates in chemical reactions under normal conditions. It does not readily form ions or covalent bonds.
However, under extreme conditions, such as high pressure or in the presence of highly reactive species, krypton can form compounds. These compounds are exceptionally rare and unstable, highlighting the strong preference for krypton to maintain its stable octet.
Applications of Krypton Leveraging its Inertness
Despite its inertness, krypton finds several applications, mostly leveraging its properties as a noble gas:
-
Lighting: Krypton is used in some types of fluorescent lamps and high-intensity discharge lamps. Its addition enhances the light output and alters the color.
-
Lasers: Krypton fluoride lasers (KrF lasers) are utilized in various applications, including semiconductor manufacturing and eye surgery. The inert nature of krypton ensures stability within the laser system.
-
Photography: Krypton-filled flash tubes are sometimes used in high-speed photography due to their intense and short-duration light emission.
-
Medical Imaging: Krypton is explored as a contrast agent for medical imaging techniques.
-
Plasma Displays: Krypton is employed in some plasma display panels for improved image quality and brightness.
Krypton Isotopes and Their Significance
Krypton has several naturally occurring isotopes, each with a different number of neutrons. The most abundant isotopes are Krypton-84 (57%), Krypton-82 (12%), and Krypton-83 (12%). The relative abundance of these isotopes can be used in various applications, including:
-
Geochronology: The ratio of certain krypton isotopes can provide insights into the age of geological samples.
-
Environmental Science: Analysis of krypton isotopes can be utilized to study atmospheric processes and pollution sources.
-
Nuclear Physics: Krypton isotopes play a role in nuclear research and applications.
Conclusion: Krypton's Valence Electrons and Their Impact
In summary, krypton possesses eight valence electrons, resulting in a highly stable electron configuration and contributing to its chemical inertness. This inertness is the key factor in its applications across various fields. While it rarely forms chemical compounds under normal conditions, its unique properties make it a valuable element with significant uses in lighting, lasers, and other technological advancements. Understanding its electron configuration allows for a comprehensive appreciation of its behavior and its vital role in scientific and industrial contexts. The future may hold even more surprising applications as researchers continue to explore the unique properties of this noble gas.
Latest Posts
Latest Posts
-
How Many Valence Electrons In Cesium
Mar 30, 2025
-
How To Find Average Velocity From Velocity Time Graph
Mar 30, 2025
-
The Water Cycle Is Driven By The
Mar 30, 2025
-
Tearing Of Paper Is A Physical Change
Mar 30, 2025
-
How Many Elements Naturally Occur On Earth
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Krypton . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
