Tearing Of Paper Is A Physical Change
News Leon
Mar 30, 2025 · 6 min read
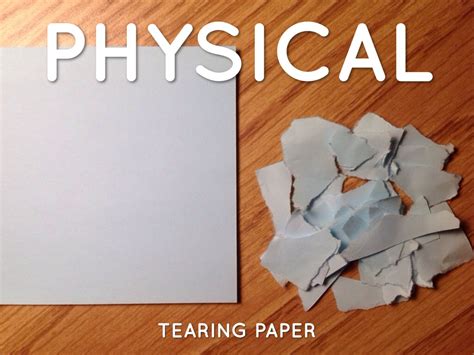
Table of Contents
Tearing Paper: A Deep Dive into Physical Changes
The simple act of tearing a piece of paper seems inconsequential, almost mundane. Yet, this everyday action provides a perfect illustration of a physical change, a fundamental concept in science. Understanding the difference between physical and chemical changes is crucial in various fields, from chemistry and physics to materials science and even cooking. This article will explore the tearing of paper as a quintessential example of a physical change, delving into the underlying principles and dispelling any common misconceptions.
What is a Physical Change?
Before we dissect the tearing of paper, let's define the term "physical change." A physical change is any alteration in the form or appearance of matter that does not result in the formation of a new substance. Crucially, the chemical composition of the material remains unchanged. Think of it like this: you're changing the shape or state of the matter, but not its fundamental building blocks. Examples of physical changes include:
- Changing state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and sublimating dry ice (solid to gas).
- Changes in shape: Bending a metal rod, tearing paper, cutting a piece of wood, crushing a can.
- Dissolving substances: Salt dissolving in water (although the resulting solution is a mixture, the salt itself hasn't changed chemically).
- Mixing substances: Combining sand and water.
The key takeaway is that in a physical change, the original substance can, in theory, be recovered through simple physical means.
Why Tearing Paper is a Physical Change
When you tear a piece of paper, you are applying a force that overcomes the intermolecular forces holding the cellulose fibers together. These fibers, composed of long chains of glucose molecules, are the primary structural component of paper. The force causes these fibers to separate, resulting in two or more pieces of paper. However, the cellulose fibers themselves remain chemically intact. The chemical composition of the paper—the actual arrangement and bonding of atoms within the cellulose molecules—has not changed.
Examining the Microscopic Level
To truly understand why tearing paper is a physical change, we need to zoom in to the microscopic level. Imagine the cellulose fibers as tiny interwoven strands. When we tear the paper, we're not breaking the chemical bonds within these individual fibers. Instead, we're disrupting the weaker forces that hold the fibers together, such as hydrogen bonds and van der Waals forces. These forces are much weaker than the covalent bonds that link the atoms within a cellulose molecule. The fibers themselves remain largely undamaged; they are simply separated.
Contrast with Chemical Changes
Let's contrast this with a chemical change. A chemical change, or chemical reaction, involves the formation of new substances with different chemical compositions. This typically involves the breaking and forming of chemical bonds. Examples include burning paper (which produces ash, carbon dioxide, and water), or the rusting of iron (which forms iron oxide).
In these instances, the original material is fundamentally altered at the molecular level. You cannot simply reverse these changes through physical means. Burning paper doesn't allow you to reassemble it into its original form, and rust cannot be readily converted back into pure iron.
Exploring the Properties of Paper
To further solidify the understanding of why tearing is a physical change, let's examine the properties of paper itself. These properties remain largely unchanged after tearing:
- Chemical Composition: The cellulose fibers remain cellulose; they don't transform into another substance.
- Density: The density of the paper, while potentially slightly altered due to surface area changes, remains fundamentally the same.
- Mass: The total mass of the paper before and after tearing remains constant, adhering to the law of conservation of mass.
- Color: The color of the paper also remains the same, unless significant fiber damage occurs, which is primarily related to the physical disruption rather than chemical alteration.
Common Misconceptions about Tearing Paper
Despite its seemingly straightforward nature, some misconceptions surround the tearing of paper:
Misconception 1: Tearing paper creates new matter.
Reality: Tearing paper only changes the shape and size of the existing matter. No new matter is created, and no matter is destroyed. The law of conservation of mass holds true.
Misconception 2: The tearing process involves chemical changes at the fiber level.
Reality: While some microscopic fiber damage might occur, the fundamental chemical composition of the cellulose fibers remains unchanged. The breakage is primarily between fibers, not within them.
Misconception 3: Tearing paper is an irreversible process.
Reality: While you cannot simply reassemble the torn pieces perfectly without adhesives, the process itself is theoretically reversible at the atomic level. The cellulose fibers remain unchanged, and with sufficient technology (though practically impossible), one could potentially re-establish the original structure. This is in stark contrast to a chemical change, which creates new substances that are irreversible by simple physical means.
The Role of Force and Energy in Tearing Paper
The act of tearing paper involves the application of external force to overcome the intermolecular forces holding the paper together. This force performs work, which translates into an energy transfer. The energy is primarily used to break the relatively weak intermolecular forces, not to alter the chemical bonds within the cellulose molecules. This energy expenditure further underscores the nature of the process as a physical, rather than chemical, change.
Real-World Applications and Implications
Understanding the physical nature of tearing paper extends beyond a simple classroom demonstration. It has implications in various fields:
- Materials Science: The study of paper's mechanical properties, including its tear strength, is crucial in designing stronger and more durable paper products.
- Recycling: The process of recycling paper relies on the fact that its chemical composition remains largely intact. The paper can be processed and reformed into new paper products.
- Forensic Science: Examining the characteristics of torn edges can be useful in forensic investigations, providing clues about the source and nature of the tear.
Conclusion: The Unassuming Physics of a Torn Piece of Paper
The seemingly simple act of tearing a piece of paper offers a powerful illustration of a physical change. It highlights the importance of distinguishing between physical and chemical changes, emphasizing that alterations in form or appearance don't always imply changes in chemical composition. By understanding the microscopic interactions and energy transfers involved, we gain a deeper appreciation for the fundamental principles governing matter and its transformations. From the classroom to the laboratory and beyond, the lesson of the torn paper reminds us of the rich physics and chemistry hidden in our everyday actions. The next time you tear a piece of paper, take a moment to consider the fascinating scientific principles at play.
Latest Posts
Latest Posts
-
D Dx X 1 X 2
Apr 01, 2025
-
Energy For Muscle Contraction Is Most Directly Supplied By
Apr 01, 2025
-
What Is The Charge Of A Sodium Ion
Apr 01, 2025
-
Do Lysosomes Have A Double Membrane
Apr 01, 2025
-
Which Of The Following Is True Of B Cells
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Tearing Of Paper Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
