What Is The Charge Of A Sodium Ion
News Leon
Apr 01, 2025 · 6 min read
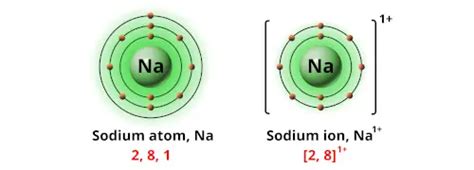
Table of Contents
What is the Charge of a Sodium Ion? Understanding Ionic Charges and Their Significance
The simple answer is: a sodium ion (Na⁺) carries a +1 charge. However, understanding why it carries this charge delves into the fascinating world of atomic structure, electron configuration, and the principles governing ionic bonding. This article will explore this fundamental concept in chemistry, examining the charge of a sodium ion in detail, and exploring its implications in various contexts.
Understanding Atomic Structure and Electron Configuration
Before we delve into the charge of a sodium ion, it's crucial to grasp the basics of atomic structure. Atoms are composed of three subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge (+1), neutrons are electrically neutral, and electrons carry a negative charge (-1). The number of protons in an atom's nucleus defines its atomic number and determines the element. For sodium (Na), the atomic number is 11, meaning it has 11 protons.
The electron configuration describes how electrons are arranged in energy levels or shells surrounding the nucleus. For sodium, the electron configuration is 1s²2s²2p⁶3s¹. This means:
- 1s²: Two electrons in the first energy level (closest to the nucleus).
- 2s²: Two electrons in the second energy level.
- 2p⁶: Six electrons in the second energy level (p-orbital).
- 3s¹: One electron in the third energy level.
This outermost electron in the 3s orbital is relatively loosely bound to the nucleus. This is because the inner electrons shield the outer electron from the full positive charge of the nucleus. This outermost electron is also called the valence electron.
Ion Formation: The Path to a +1 Charge
Atoms are most stable when their outermost electron shell is full. For sodium, having only one electron in its outermost shell makes it relatively unstable. To achieve stability, sodium readily loses this single valence electron. This process is called ionization.
When sodium loses its valence electron, it no longer has an equal number of protons and electrons. It now has 11 protons (positive charges) and 10 electrons (negative charges). The difference results in a net positive charge of +1. This positively charged sodium atom is now called a sodium ion, represented as Na⁺.
The Role of Electronegativity
Electronegativity is the measure of an atom's ability to attract electrons towards itself in a chemical bond. Sodium has a relatively low electronegativity. This means it has a weaker hold on its valence electron compared to elements with higher electronegativity, like chlorine or oxygen. This low electronegativity contributes to sodium's willingness to lose its electron and form a positive ion.
Contrasting with other ions
It is important to contrast sodium's behavior with that of other elements. For instance, chlorine (Cl) has seven valence electrons. To achieve a stable octet, it readily gains one electron, becoming a negatively charged chloride ion (Cl⁻). This illustrates how the drive for a stable electron configuration dictates ion formation and charge.
Ionic Compounds: The Result of Ionic Bonding
Sodium ions rarely exist in isolation. Their positive charge attracts negatively charged ions, leading to the formation of ionic compounds. A classic example is sodium chloride (NaCl), or common table salt. In NaCl, each sodium ion (Na⁺) is electrostatically attracted to a chloride ion (Cl⁻), forming a crystalline structure. The overall compound is electrically neutral because the positive and negative charges balance each other.
Implications of the +1 Charge in Ionic Compounds
The +1 charge of the sodium ion is crucial in determining the stoichiometry (ratio of elements) in ionic compounds. Since sodium has a +1 charge and chloride has a -1 charge, they combine in a 1:1 ratio to form NaCl. If sodium were to react with an element that forms a -2 ion (like oxygen), the formula would be Na₂O, reflecting the need for two sodium ions to balance the charge of one oxide ion.
Sodium Ion in Biological Systems
Sodium ions play a vital role in numerous biological processes. Their +1 charge enables them to participate in:
- Nerve impulse transmission: Sodium ions flow across nerve cell membranes, creating electrical signals that transmit information throughout the body. The movement of these ions is tightly regulated by ion channels.
- Muscle contraction: Similar to nerve impulses, sodium ions are essential for muscle contraction. Changes in sodium ion concentrations trigger the release of calcium, leading to muscle fiber contraction.
- Fluid balance: Sodium ions help regulate the amount of water in the body. They contribute to osmotic pressure, maintaining the proper balance of fluids inside and outside cells.
- Nutrient absorption: Sodium ions assist in the absorption of nutrients from the digestive system into the bloodstream.
The precise concentration and movement of sodium ions are strictly controlled within living organisms. Dysregulation of sodium ion levels can have significant consequences on health, leading to conditions like hyponatremia (low sodium) or hypernatremia (high sodium).
Industrial Applications of Sodium Compounds
The unique properties of sodium ions and their compounds make them valuable in various industrial applications. Examples include:
- Salt production: Sodium chloride (NaCl) is a staple in numerous industrial processes, ranging from food preservation to water softening.
- Soap production: Sodium hydroxide (NaOH) is a key ingredient in soap manufacturing. Its strong base properties help convert fats and oils into soap molecules.
- Paper manufacturing: Sodium compounds play roles in pulping, bleaching, and sizing paper.
- Glass manufacturing: Sodium compounds are essential components in the production of glass, contributing to its durability and other properties.
Analytical Techniques for Detecting Sodium Ions
Several techniques can detect and measure sodium ions:
- Flame photometry: When sodium ions are introduced into a flame, they emit light at a characteristic wavelength. The intensity of this light is proportional to the concentration of sodium ions.
- Atomic absorption spectroscopy (AAS): This technique measures the amount of light absorbed by sodium atoms in a sample. The absorbance is directly related to the concentration of sodium ions.
- Ion-selective electrodes (ISEs): These electrodes are specifically designed to measure the concentration of sodium ions in a solution. They use a membrane that is selectively permeable to sodium ions.
These techniques are crucial in various settings, such as clinical chemistry (measuring sodium levels in blood), environmental monitoring (detecting sodium in water samples), and industrial quality control.
Conclusion: The Significance of a Simple Charge
The seemingly simple +1 charge of a sodium ion has profound implications across various fields. Its understanding is fundamental to comprehending ionic bonding, biological processes, and industrial applications. From the intricate workings of nerve impulses to the production of everyday materials, the presence and behavior of sodium ions are ubiquitous and crucial to the world around us. This article has explored the charge of a sodium ion, its formation, its role in various processes, and methods for its detection. By understanding this basic yet fundamental aspect of chemistry, we gain a deeper appreciation for the intricacies of the natural and man-made worlds.
Latest Posts
Latest Posts
-
All Summer In A Day Ray Bradbury Summary
Apr 02, 2025
-
Equal Forces Acting In Opposite Directions
Apr 02, 2025
-
What Volume In Of A Solution Contains
Apr 02, 2025
-
Calculate The Binding Energy Per Nucleon
Apr 02, 2025
-
Oxidation Number Of Iron In Fe3o4
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of A Sodium Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
