How Many Elements Naturally Occur On Earth
News Leon
Mar 30, 2025 · 6 min read
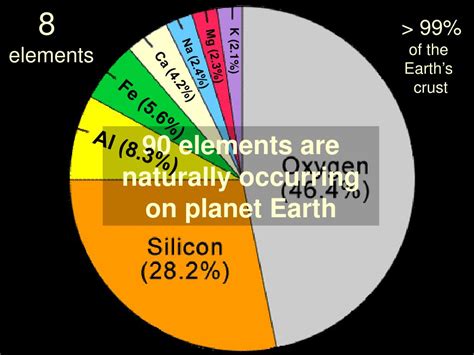
Table of Contents
How Many Elements Naturally Occur on Earth? A Deep Dive into the Periodic Table's Terrestrial Representatives
The Earth, our vibrant and diverse planet, is a treasure trove of chemical elements. But how many of these fundamental building blocks of matter naturally occur here? The answer isn't a simple number, and understanding the nuances requires a journey into the fascinating world of chemistry and geology.
The Periodic Table and Terrestrial Abundance
The periodic table organizes all known elements, showcasing their properties and relationships. However, simply counting the elements present on Earth doesn't paint the full picture. We need to consider several factors:
- Naturally Occurring: This refers to elements found in nature without human intervention, excluding those created artificially in laboratories.
- Abundance: Some elements are ubiquitous, while others are incredibly rare. This impacts their significance in Earth's composition and processes.
- Isotopes: Elements exist as isotopes – atoms with the same number of protons but differing numbers of neutrons. The stability of these isotopes greatly influences their presence.
While the periodic table lists over 118 elements, the number naturally occurring on Earth is significantly less. The precise count is a matter of debate, depending on the criteria used.
The Big Players: Abundant Elements Shaping Our World
A small number of elements dominate Earth's composition. These are crucial for understanding our planet's geology, atmosphere, and even life itself.
Oxygen (O): The Dominant Force
Oxygen reigns supreme, making up about 46.6% of the Earth's crust by mass. This reactive element is essential for respiration in most living organisms and forms a crucial component of water and numerous minerals. Its presence in the atmosphere, as O₂, is vital for sustaining life as we know it.
Silicon (Si): The Backbone of Rocks
Silicon comes in second, representing around 27.7% of the crust. It forms the backbone of silicate minerals, the primary constituents of most rocks. Silicon's unique bonding properties allow for the creation of complex and diverse geological structures.
Aluminum (Al): Lightweight and Abundant
Aluminum contributes approximately 8.1% to the Earth's crust. This lightweight yet strong metal is widely used in various applications due to its abundance and properties.
Iron (Fe): The Core Component
Iron, while less prevalent in the crust (around 5%), dominates Earth's core. This element's magnetic properties are responsible for Earth's protective magnetic field, shielding us from harmful solar radiation.
Other Major Components
Other elements like calcium (Ca), sodium (Na), potassium (K), magnesium (Mg), and titanium (Ti) make up significant portions of the Earth's crust. These elements are crucial components of various minerals and play vital roles in geological processes.
The Less Abundant, Yet Crucial Elements
While the elements mentioned above dominate Earth's composition by mass, numerous other elements contribute significantly to the planet's diversity and functionality. Many of these, despite their lower overall abundance, are essential for biological processes or technological advancements.
Trace Elements: Small Amounts, Big Impact
Trace elements, present in minute quantities, are often vital for life. These include:
- Copper (Cu): Essential for enzyme function in many organisms.
- Zinc (Zn): Crucial for numerous enzymatic reactions and protein synthesis.
- Manganese (Mn): Important for plant growth and enzyme activity.
- Cobalt (Co): A component of vitamin B12, essential for human health.
- Molybdenum (Mo): Involved in nitrogen fixation by certain bacteria.
- Iodine (I): Essential for thyroid hormone production.
- Selenium (Se): An antioxidant with crucial roles in various metabolic processes.
The importance of these trace elements highlights the intricate interplay of even the least abundant elements within Earth's ecosystems. Deficiencies in these elements can have severe consequences for living organisms.
Radioactive Elements and Their Significance
Radioactive elements, characterized by their unstable nuclei, play a significant role in understanding Earth's age and geological processes. These elements decay over time, emitting radiation and transforming into different elements.
- Uranium (U) and Thorium (Th): These elements are relatively abundant and contribute significantly to Earth's internal heat, driving plate tectonics and volcanic activity. Their radioactive decay is also used for radiometric dating, allowing scientists to determine the age of rocks and minerals.
- Potassium-40 (⁴⁰K): This radioactive isotope of potassium contributes to Earth's heat production and is used in dating older rocks.
- Carbon-14 (¹⁴C): While less abundant, this isotope is crucial for radiocarbon dating, a technique used to date organic materials.
The Rare Earth Elements: A Technological Treasure
Rare earth elements (REEs) comprise a group of 17 elements – the lanthanides plus scandium and yttrium. These elements, despite their name, aren't necessarily rare in terms of absolute abundance but are challenging to extract and process economically. Their unique magnetic, luminescent, and catalytic properties make them crucial for many modern technologies, including smartphones, wind turbines, and hybrid vehicles.
Their scarcity in easily accessible, concentrated deposits presents significant geopolitical and environmental challenges. Securing a stable supply of REEs is a growing concern for many nations.
Synthesized Elements: Outside the Natural Realm
It's important to differentiate between naturally occurring elements and those created artificially. Elements with atomic numbers beyond 94 (Plutonium) are primarily synthesized in particle accelerators. These elements are not naturally found on Earth, at least not in significant quantities, and are highly unstable.
The Elusive Count: Defining "Naturally Occurring"
Determining the exact number of naturally occurring elements is a complex task. The definition of "naturally occurring" can be debated:
- Trace Amounts: Some elements are present in such minuscule quantities that their detection and confirmation can be difficult.
- Cosmogenic Nuclides: Certain isotopes are created by cosmic ray interactions with the atmosphere. Including these expands the number of elements considered naturally occurring.
- Geochemical Processes: Geological processes can concentrate or disperse elements, making their presence highly localized.
Considering these factors, a conservative estimate of naturally occurring elements on Earth falls within the range of 90 to 98. This number represents those elements found in measurable quantities and not solely created artificially. However, the precise count continues to be refined as analytical techniques improve and our understanding of Earth's composition deepens.
Conclusion: A Dynamic and Ever-Evolving Inventory
The Earth's elemental inventory is far from static. Geological processes, radioactive decay, and even human activities continuously reshape the distribution and availability of elements. The interplay between abundant and trace elements, radioactive isotopes, and rare earth elements defines our planet's character, drives its geological evolution, and supports life's remarkable diversity. While pinning down a precise number of naturally occurring elements remains a challenge, the quest to understand their distribution, abundance, and interactions continues to be a fundamental pursuit in scientific exploration. This dynamic inventory holds the key to understanding our planet's past, present, and future.
Latest Posts
Latest Posts
-
What Is Not A Product Of Photosynthesis
Apr 01, 2025
-
D Dx X 1 X 2
Apr 01, 2025
-
Energy For Muscle Contraction Is Most Directly Supplied By
Apr 01, 2025
-
What Is The Charge Of A Sodium Ion
Apr 01, 2025
-
Do Lysosomes Have A Double Membrane
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Elements Naturally Occur On Earth . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
