How Many Valence Electrons In Cesium
News Leon
Mar 30, 2025 · 5 min read
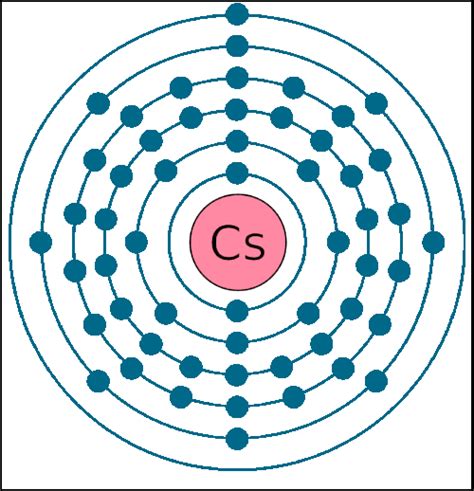
Table of Contents
How Many Valence Electrons Does Cesium Have? Unraveling the Mysteries of Alkali Metals
Cesium, a fascinating element residing in the alkali metal family, holds a unique place in the periodic table. Understanding its electronic structure, particularly the number of valence electrons, is crucial to comprehending its chemical behavior and reactivity. This comprehensive guide delves deep into the world of cesium, explaining not only the number of its valence electrons but also the implications of this number on its properties and applications.
Understanding Valence Electrons: The Key to Chemical Behavior
Before we dive into cesium specifically, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary players in chemical bonding, dictating how an atom will interact with other atoms to form molecules and compounds. The number of valence electrons directly influences an element's reactivity, bonding tendencies, and overall chemical properties. Elements with similar numbers of valence electrons often exhibit similar chemical behaviors, a pattern clearly reflected in the periodic table's organization.
The Electronic Configuration of Cesium: Unveiling the Outermost Shell
Cesium (Cs), with an atomic number of 55, possesses 55 protons and, in its neutral state, 55 electrons. To determine the number of valence electrons, we need to examine its electronic configuration. The electronic configuration describes how electrons are distributed across different energy levels and subshells within an atom. For cesium, the electronic configuration is:
1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 6s¹
This configuration reveals the arrangement of electrons in various shells. The outermost shell, in this case, is the sixth shell (n=6). Notice that the sixth shell contains only one electron in the 6s subshell.
The Answer: Cesium Possesses One Valence Electron
Based on the electronic configuration, the definitive answer is: Cesium (Cs) has one valence electron. This single valence electron residing in the 6s orbital is responsible for cesium's characteristic chemical properties and its high reactivity.
Implications of One Valence Electron: Reactivity and Bonding
Having only one valence electron profoundly influences cesium's chemical behavior. Atoms strive for stability, often achieved by having a full outermost electron shell. Because cesium only needs to lose one electron to achieve a stable configuration (like the noble gas Xenon), it readily does so. This tendency to lose an electron makes cesium highly reactive, particularly with electronegative elements like halogens (fluorine, chlorine, bromine, iodine).
Ionic Bonding:
Cesium's most common type of bonding is ionic bonding. It readily loses its valence electron to form a +1 cation (Cs⁺). This positively charged ion then forms ionic compounds with negatively charged anions, such as chloride (Cl⁻) in cesium chloride (CsCl) or iodide (I⁻) in cesium iodide (CsI). The electrostatic attraction between the oppositely charged ions creates the ionic bond.
Metallic Bonding:
In its pure elemental form, cesium exhibits metallic bonding. The valence electrons are delocalized, forming a "sea" of electrons that are shared among the positively charged cesium ions. This delocalization accounts for cesium's characteristic metallic properties like conductivity, malleability, and ductility.
Reactivity with Water:
Cesium's high reactivity is famously showcased in its reaction with water. It reacts violently, producing hydrogen gas and cesium hydroxide. The reaction is highly exothermic, often resulting in ignition of the hydrogen gas. This extreme reactivity emphasizes the cesium atom's strong desire to lose its single valence electron to achieve stability.
Cesium's Applications: Leveraging its Unique Properties
The unique properties stemming from its single valence electron make cesium valuable in various applications:
-
Atomic Clocks: Cesium's precise atomic transitions are used in atomic clocks, providing highly accurate timekeeping. The cesium atom's response to specific microwave frequencies is exploited to define the second – the fundamental unit of time.
-
Photoelectric Cells: Cesium's low ionization energy means it readily emits electrons when exposed to light. This property is utilized in photoelectric cells, which convert light into electricity. These cells find applications in various devices, including light meters and solar cells (although other elements are more commonly used in commercially available solar cells).
-
Oil Exploration: Cesium compounds are used in oil exploration for logging and formation evaluation techniques. They aid in determining reservoir properties and guiding drilling operations.
-
Catalysis: Cesium compounds can act as catalysts in certain chemical reactions, facilitating faster and more efficient processes.
-
Medical Imaging (limited): While less common compared to other elements, some cesium compounds have been explored for their potential in medical imaging applications, though they aren't widely used in standard procedures.
Comparison with Other Alkali Metals: Similarities and Differences
Cesium belongs to Group 1 of the periodic table, the alkali metals. All alkali metals share the characteristic of having one valence electron, leading to similar chemical properties: high reactivity, ease of forming +1 cations, and metallic bonding in their pure elemental state.
However, there are differences in reactivity. Cesium is the most reactive alkali metal, a direct consequence of its large atomic size and the resulting greater distance between the nucleus and its single valence electron. This weak attraction allows for easier loss of the electron, leading to its exceptionally high reactivity. Lithium, sodium, potassium, rubidium, and francium, the other alkali metals, are also reactive, but less so than cesium.
Conclusion: The Significance of Cesium's Single Valence Electron
The single valence electron in cesium is the cornerstone of its distinctive properties and applications. This electron's behavior dictates cesium's high reactivity, its tendency to form ionic compounds, its role in metallic bonding, and its suitability for various technological applications. Understanding the significance of this single electron provides a comprehensive appreciation for cesium's unique position in the chemical world, highlighting its importance in scientific advancements and technological innovations. From precise atomic clocks to specialized industrial applications, cesium's one valence electron plays a crucial role in shaping its impact on our world. The seemingly simple fact that cesium possesses just one valence electron holds a profound impact on its chemical behavior and the diverse roles it plays in modern technology and scientific research. Further exploration into the intricacies of its electronic structure and chemical reactions continues to reveal fascinating aspects of this remarkable element.
Latest Posts
Latest Posts
-
Energy For Muscle Contraction Is Most Directly Supplied By
Apr 01, 2025
-
What Is The Charge Of A Sodium Ion
Apr 01, 2025
-
Do Lysosomes Have A Double Membrane
Apr 01, 2025
-
Which Of The Following Is True Of B Cells
Apr 01, 2025
-
How Many Parents Are Involved In Asexual Reproduction
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Cesium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
