How Many Valence Electrons Are In Argon
News Leon
Mar 23, 2025 · 5 min read
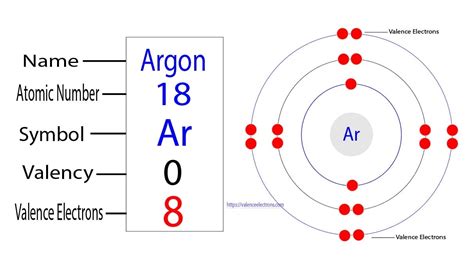
Table of Contents
How Many Valence Electrons Are in Argon? A Deep Dive into Atomic Structure
Argon, a noble gas celebrated for its inert nature, holds a unique position in the periodic table. Understanding its electronic configuration, particularly the number of valence electrons, is crucial to grasping its chemical behavior and its applications in various fields. This comprehensive guide will delve into the intricacies of argon's atomic structure, explaining why it possesses a full valence shell and the implications of this characteristic.
Understanding Valence Electrons
Before focusing on argon, let's establish a solid foundation on the concept of valence electrons. Valence electrons are the electrons located in the outermost shell of an atom. These electrons are the primary players in chemical bonding, determining an element's reactivity and the types of bonds it can form. The number of valence electrons significantly impacts an element's chemical properties. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to fill their outermost shell. This stability is often associated with the "octet rule," which suggests that atoms tend to gain, lose, or share electrons until they have eight electrons in their valence shell. However, it's important to note that the octet rule is not universally applicable, especially for elements beyond the second period.
Electron Shells and Subshells
Atoms possess electrons arranged in various shells and subshells. The principal quantum number (n) designates the electron shell, with n=1 representing the shell closest to the nucleus. Each shell can hold a maximum number of electrons, calculated using the formula 2n². Within each shell, we find subshells, designated by the letters s, p, d, and f. Each subshell can hold a specific number of electrons: s (2 electrons), p (6 electrons), d (10 electrons), and f (14 electrons).
Argon's Electronic Configuration
Argon (Ar) has an atomic number of 18, meaning it possesses 18 protons and 18 electrons in its neutral state. To determine its electronic configuration, we systematically fill the electron shells and subshells following the Aufbau principle and Hund's rule. The Aufbau principle dictates that electrons fill the lowest energy levels first, while Hund's rule states that electrons individually occupy each orbital within a subshell before pairing up.
The electronic configuration of argon is 1s²2s²2p⁶3s²3p⁶. Let's break this down:
- 1s²: Two electrons occupy the 1s subshell (the lowest energy level).
- 2s²: Two electrons occupy the 2s subshell.
- 2p⁶: Six electrons occupy the 2p subshell.
- 3s²: Two electrons occupy the 3s subshell.
- 3p⁶: Six electrons occupy the 3p subshell.
This configuration signifies that argon's three shells are completely filled. The first shell holds its maximum of two electrons, the second shell holds its maximum of eight electrons (2s² + 2p⁶), and the third shell, its outermost shell, also holds its maximum of eight electrons (3s² + 3p⁶).
Determining Argon's Valence Electrons
Given argon's electronic configuration, identifying its valence electrons becomes straightforward. The valence electrons are those in the outermost shell, which, in argon's case, is the third shell. This shell contains eight electrons (3s²3p⁶). Therefore, argon has eight valence electrons.
Argon's Inertness and Full Valence Shell
The presence of eight valence electrons explains argon's inertness – its reluctance to participate in chemical reactions. A completely filled outermost shell represents a state of high stability. Argon doesn't need to gain, lose, or share electrons to achieve a more stable configuration; it already possesses the maximum number of electrons its outermost shell can accommodate. This stable octet configuration contributes significantly to its chemical inactivity.
Argon's Applications: Leveraging its Inertness
Argon's inertness makes it invaluable in various applications where preventing unwanted chemical reactions is crucial. Some key applications include:
- Welding: Argon's inert atmosphere protects the weld from oxidation and contamination. This ensures a stronger and more reliable weld.
- Metal Production: In metallurgy, argon shields reactive metals from atmospheric gases during processing, preventing oxidation and preserving the metal's purity.
- Light Bulbs: Argon is used in incandescent light bulbs to prevent the filament from oxidizing and burning out prematurely, extending the bulb's lifespan.
- Scientific Research: Its inert nature makes argon useful in various scientific instruments and processes where reactive environments must be avoided.
- Healthcare: Argon laser treatment is employed in various surgical procedures.
Comparing Argon to Other Noble Gases
Argon shares its inertness with other noble gases (helium, neon, krypton, xenon, radon, and oganesson). They all possess a full valence shell, contributing to their low reactivity. However, their atomic sizes and electronic configurations differ, leading to variations in their physical and some chemical properties. While all are generally unreactive, heavier noble gases can participate in some chemical reactions under specific conditions.
The Exception: Helium
Helium, with only two electrons (1s²), is an exception to the octet rule. Its outermost shell is complete with two electrons, achieving stability. This illustrates that a full outermost shell, regardless of the number of electrons it contains, equates to stability for all noble gases.
Conclusion: Argon's Significance in Chemistry and Beyond
Argon's eight valence electrons define its chemical inertness, which is the cornerstone of its diverse applications. Understanding the relationship between electronic configuration, valence electrons, and chemical reactivity provides a comprehensive perspective on argon's role in science and technology. Its inertness makes it an indispensable element in various industrial processes, safeguarding materials from unwanted reactions and preserving their integrity. The stable electronic configuration of argon serves as a testament to the principles governing atomic structure and chemical bonding. Further exploration into the properties and applications of noble gases will undoubtedly unveil even more of their scientific significance and technological potential. The ubiquitous presence of argon highlights its essential role in many aspects of modern life.
Latest Posts
Latest Posts
-
Definition Of Order Of A Reaction
Mar 25, 2025
-
Distilled Water Does Not Conduct A Current
Mar 25, 2025
-
A Projectile Is Fired Horizontally From A Gun
Mar 25, 2025
-
Why Europe Is Called The Peninsula Of Peninsulas
Mar 25, 2025
-
Which Of The Following Is A Mineralocorticoid
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Argon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
