Boiling Water Is A Physical Change
News Leon
Mar 27, 2025 · 5 min read
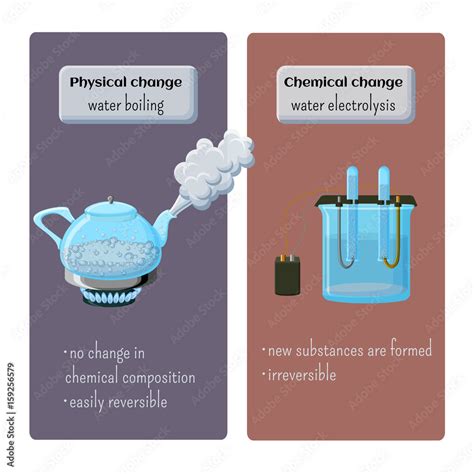
Table of Contents
Boiling Water is a Physical Change: A Deep Dive into the Science
Boiling water is a quintessential example of a physical change, not a chemical change. While it might seem dramatic – the water transforms from a liquid to a gas – the fundamental chemical composition remains unchanged. This article will delve into the science behind this phenomenon, exploring the concepts of physical and chemical changes, the properties of water, and the intricacies of the boiling process. We'll also address common misconceptions and provide further examples to solidify your understanding.
Understanding Physical and Chemical Changes
Before we dive into the specifics of boiling water, let's establish a clear understanding of the difference between physical and chemical changes. This distinction is crucial for grasping the nature of boiling.
Physical Changes: These changes affect the form or appearance of a substance but do not alter its chemical composition. The substance remains the same molecule, simply in a different state or form. Examples include:
- Changes of state: Melting, freezing, boiling, condensation, sublimation (solid to gas), and deposition (gas to solid).
- Shape changes: Cutting, bending, crushing.
- Dissolving (in some cases): Salt dissolving in water is a physical change because the salt molecules are still present; they're just dispersed in the water.
Chemical Changes: These changes alter the chemical composition of a substance, resulting in the formation of a new substance with different properties. Chemical reactions are involved, often indicated by changes in temperature, color, odor, or the formation of a precipitate (solid). Examples include:
- Burning: Wood burning into ash and gases.
- Rusting: Iron reacting with oxygen to form iron oxide.
- Cooking: Many cooking processes involve chemical changes, such as the browning of meat.
The Boiling Process: A Detailed Look
Boiling water is a classic example of a physical change because it involves a change of state – from liquid water (H₂O) to gaseous water (water vapor or steam). The chemical formula remains H₂O throughout the process. No new substance is created.
What Happens When Water Boils?
When heat is applied to liquid water, the water molecules absorb energy. This energy increases the kinetic energy of the molecules, causing them to move faster and faster. At a certain temperature, the kinetic energy overcomes the intermolecular forces holding the water molecules together in the liquid state.
This temperature is the boiling point, which is 100°C (212°F) at standard atmospheric pressure. At this point, the molecules gain enough energy to escape the liquid phase and transition to the gaseous phase, forming water vapor (steam). This phase transition is characterized by vigorous bubbling and the formation of steam.
Key Characteristics of Boiling as a Physical Change:
- Reversibility: The process is easily reversible. By cooling the steam, it condenses back into liquid water. This demonstrates that no new substance was formed during boiling.
- No new substance formation: The chemical composition remains unchanged. The steam is still water (H₂O), just in a different state.
- No significant energy change (excluding latent heat): While energy is required to boil water (latent heat of vaporization), this energy is not used to create new chemical bonds or break existing ones. The energy is used to overcome the intermolecular forces between water molecules.
- Separation, not reaction: Boiling can be viewed as a separation process. The water molecules are separating from each other, not reacting to form new molecules.
Addressing Common Misconceptions
Despite the clear scientific evidence, some misconceptions persist regarding boiling water. Let's clarify them:
Misconception 1: Boiling water changes its chemical properties.
Reality: The chemical composition of water (H₂O) remains unchanged. While the physical properties (like density and volume) change, the water molecule remains the same.
Misconception 2: The steam produced is different from water.
Reality: Steam is simply water in its gaseous state. It's still H₂O, just spread out more. The difference lies in the physical state, not the chemical composition.
Misconception 3: Boiling water involves a chemical reaction.
Reality: Boiling is a purely physical process. No new chemical bonds are formed or broken. It's a change of state, not a chemical reaction.
Further Examples of Physical Changes
To solidify the understanding of physical changes, here are some additional examples that share similarities with the boiling of water:
- Melting ice: Ice (solid water) melts into liquid water. The chemical composition remains the same; only the state changes.
- Freezing water: Liquid water turns into solid ice. Again, only the physical state changes.
- Sublimation of dry ice: Solid carbon dioxide transitions directly to a gas without becoming a liquid. The CO2 molecule remains the same.
- Condensation of water vapor: Water vapor cools and turns back into liquid water. A reversible process, showing no chemical alteration.
- Dissolving sugar in water: Sugar molecules disperse in water, but they retain their chemical structure. They can be recovered by evaporating the water.
Practical Applications and Significance
Understanding that boiling water is a physical change has several significant practical applications:
- Water purification: While boiling doesn't remove all contaminants, it effectively kills many harmful bacteria and microorganisms due to the heat.
- Cooking: Boiling is a fundamental cooking method used for various purposes, from cooking vegetables to sterilizing food.
- Steam generation: Steam is used for various industrial processes, including power generation and sterilization.
- Distillation: Boiling and condensation are key processes in distillation, a method used to separate liquids with different boiling points.
Conclusion: The Simplicity and Significance of Boiling Water
Boiling water, a seemingly simple phenomenon, serves as a powerful example of a physical change. Its understanding underscores the importance of distinguishing between physical and chemical transformations. The reversibility of the process, the unchanged chemical composition, and the lack of new substance formation clearly demonstrate its physical nature. This knowledge has vast implications across various fields, highlighting the significance of this fundamental process in both everyday life and advanced scientific applications. By comprehending the science behind boiling water, we gain a deeper appreciation for the intricate world of matter and its transformations.
Latest Posts
Latest Posts
-
17 Protons 18 Neutrons 17 Electrons
Mar 30, 2025
-
Is Melting Of Wax A Physical Or Chemical Change
Mar 30, 2025
-
Examples Of Input And Output Process
Mar 30, 2025
-
How Many Electrons Does Antimony Have
Mar 30, 2025
-
Pericardial Fluid Is Found Between The And The
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
