Is Melting Of Wax A Physical Or Chemical Change
News Leon
Mar 30, 2025 · 5 min read
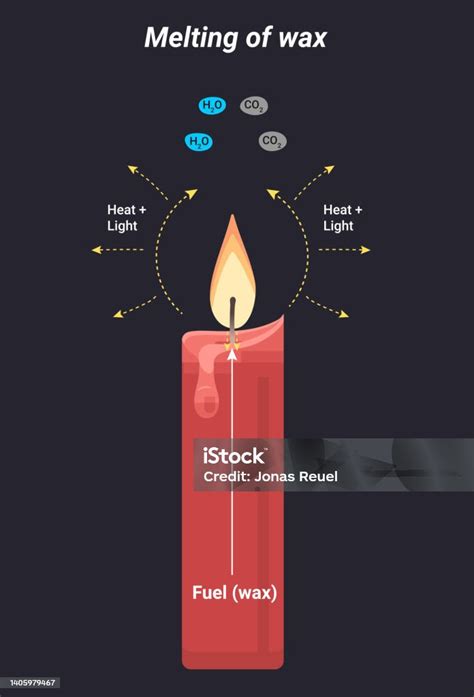
Table of Contents
Is Melting Wax a Physical or Chemical Change? A Comprehensive Exploration
The simple act of melting wax, a common occurrence in candle-making and various other applications, raises a fundamental question in chemistry: is this a physical or chemical change? The answer, as we'll explore in depth, is physical. However, understanding why requires a deeper dive into the nature of physical and chemical changes, the properties of waxes, and the processes involved in melting.
Understanding Physical and Chemical Changes
Before we tackle the wax conundrum, let's establish clear definitions:
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. The molecules remain the same; only their arrangement or state of matter changes. Examples include melting ice, dissolving sugar in water, or bending a piece of metal. These changes are often reversible.
Chemical Change: A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. These changes are often irreversible and are accompanied by indicators like a change in color, temperature, or the formation of a gas or precipitate. Examples include burning wood, rusting iron, or cooking an egg.
The Composition and Properties of Wax
Waxes are complex mixtures of long-chain hydrocarbons, esters, and other organic compounds. The specific composition varies significantly depending on the source (beeswax, paraffin wax, soy wax, etc.). However, their shared characteristic is their high molecular weight and relatively strong intermolecular forces, primarily van der Waals forces. These forces hold the molecules together in a solid structure at room temperature.
Different types of waxes possess distinct properties influencing their melting points, hardness, and other characteristics relevant to their applications. For instance:
- Paraffin wax, derived from petroleum, is a relatively inexpensive and widely used wax known for its clean burning properties.
- Beeswax, a natural wax secreted by honeybees, is prized for its unique scent and properties, often used in cosmetics and candles.
- Soy wax, a vegetable-based wax, is gaining popularity due to its environmentally friendly nature and longer burn time.
The Melting Process: A Microscopic Perspective
When wax is heated, the added thermal energy increases the kinetic energy of its molecules. This increased kinetic energy overcomes the intermolecular forces holding the molecules in a rigid, solid structure. The molecules gain more freedom of movement, transitioning from a tightly packed, ordered arrangement to a more disordered, fluid state – liquid wax.
Crucially, the chemical bonds within the individual wax molecules remain intact throughout this process. No new molecules are formed, and no existing molecules are broken down. The only change is the physical state of the wax, from solid to liquid. This is why melting wax is classified as a physical change.
Reversible Nature: Solidification
Further emphasizing the physical nature of the change, the melting process is completely reversible. Upon cooling, the wax molecules lose kinetic energy, allowing the intermolecular forces to reassert their dominance. The molecules reorganize themselves into a solid structure, and the wax solidifies. This reversibility is a hallmark of physical changes.
Differentiating from Chemical Changes Involving Wax
While melting wax is a purely physical process, other interactions with wax can involve chemical changes. Consider the following examples:
Combustion (Burning)
Burning a candle, seemingly a simple process, is actually a complex chemical reaction. The wax undergoes combustion, reacting with oxygen in the air to produce carbon dioxide, water vapor, and heat. This involves the breaking and formation of chemical bonds, resulting in entirely new substances with different properties. This is a chemical change.
Oxidation
Over time, wax can undergo oxidation, a slow reaction with oxygen that can alter its chemical composition, particularly its color and odor. This process is typically accelerated by exposure to sunlight and heat. While the primary melting process is physical, long-term storage may introduce minor chemical changes.
Interaction with Additives
When additives are incorporated into wax, such as dyes, fragrances, or other chemicals, potential chemical interactions can occur. The nature and extent of these reactions depend heavily on the specific additives used. Some additives may only cause a physical mixture, while others might react chemically with the wax components.
Practical Applications and Implications
Understanding the distinction between physical and chemical changes in the context of wax has several practical implications:
-
Candle Making: Knowing that melting wax is a physical change allows candle makers to precisely control the melting and solidifying process to create candles with desired shapes and properties. The reversibility of the process is essential for shaping and reusing wax.
-
Cosmetics and Pharmaceuticals: Waxes are used extensively in cosmetics and pharmaceuticals as emulsifiers, binders, and consistency agents. Understanding their behavior under different temperatures is crucial for product development and stability.
-
Industrial Applications: Waxes are used in various industrial applications, such as coatings, lubricants, and mold release agents. Their physical properties, particularly their melting points and viscosity, are key factors in selecting the appropriate wax for a given application.
Conclusion: The Irrefutable Physical Nature of Wax Melting
In conclusion, the melting of wax is unequivocally a physical change. The process involves only a change in the physical state of the wax, from solid to liquid, without altering its chemical composition. The molecules remain the same; only their arrangement and energy levels change. While other processes involving wax, such as burning or oxidation, can involve chemical changes, the simple act of melting remains a purely physical phenomenon, entirely reversible and governed by the principles of intermolecular forces and thermal energy. Understanding this distinction is fundamental to a variety of applications and underscores the importance of grasping the basic concepts of physical and chemical changes in chemistry.
Latest Posts
Latest Posts
-
What Is The Opposite Of Brilliant
Apr 01, 2025
-
Does Accumulated Depreciation Have A Credit Balance
Apr 01, 2025
-
What Element Has 4 Protons And 5 Neutrons
Apr 01, 2025
-
Why Does Radius Decrease Across A Period
Apr 01, 2025
-
The Krebs Cycle Takes Place In
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Of Wax A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
