Why Does Radius Decrease Across A Period
News Leon
Apr 01, 2025 · 5 min read
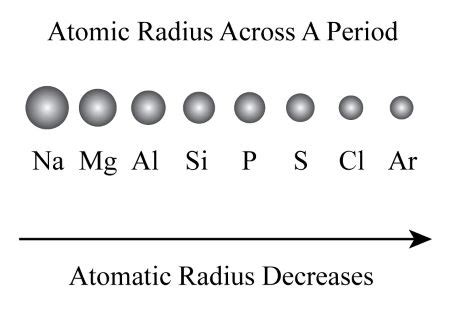
Table of Contents
Why Does Atomic Radius Decrease Across a Period? A Deep Dive into Periodic Trends
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic structure and resulting properties. One of the key periodic trends is the decrease in atomic radius across a period (from left to right). Understanding why this happens requires a closer look at the fundamental forces within an atom. This article will delve into the reasons behind this decrease, exploring the roles of effective nuclear charge and electron shielding. We'll also examine exceptions and nuances within this trend.
Understanding Atomic Radius
Before exploring the reasons for the decrease, let's define what we mean by atomic radius. Atomic radius refers to the distance from the atom's nucleus to its outermost electron shell. It's important to remember that the electron cloud doesn't have a definite boundary; the radius is often defined as half the distance between the nuclei of two identical atoms bonded together. Therefore, measuring atomic radius is not a straightforward process, and different methods can yield slightly different values. However, the overall trend remains consistent.
The Dominant Factor: Effective Nuclear Charge
The primary reason for the decrease in atomic radius across a period is the increase in effective nuclear charge. Let's break down this crucial concept:
-
Nuclear Charge: This refers to the positive charge of the nucleus, which is equal to the number of protons. As you move across a period, the number of protons increases.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. They partially cancel out the positive charge of the protons.
-
Effective Nuclear Charge (Zeff): This is the net positive charge experienced by an electron after accounting for the shielding effect of other electrons. It's calculated as the difference between the nuclear charge and the shielding effect.
The key takeaway is that as you move across a period, the number of protons increases, significantly increasing the nuclear charge. While the number of electrons also increases, they are added to the same principal energy level (shell). The increase in nuclear charge outweighs the increase in shielding, leading to a higher effective nuclear charge (Zeff).
This stronger effective nuclear charge pulls the outer electrons closer to the nucleus, resulting in a smaller atomic radius.
Illustrative Example: Comparing Sodium (Na) and Chlorine (Cl)
Let's compare sodium (Na), an alkali metal at the beginning of period 3, and chlorine (Cl), a halogen at the end of the same period.
-
Sodium (Na): Has 11 protons and 11 electrons. The outermost electron is relatively far from the nucleus, experiencing less effective nuclear charge.
-
Chlorine (Cl): Has 17 protons and 17 electrons. The outermost electrons are pulled much closer to the nucleus due to a much higher effective nuclear charge. The added electrons are in the same energy level, providing minimal shielding.
The Role of Electron Shielding
While the increase in effective nuclear charge is the dominant factor, the shielding effect plays a crucial role. Inner electrons, those in lower energy levels closer to the nucleus, partially shield the outer electrons from the full positive charge of the nucleus. However, electrons within the same principal energy level (shell) provide very little shielding.
As we move across a period, electrons are added to the same energy level (e.g., the 3rd energy level for Period 3). These electrons don't effectively shield each other from the increasing nuclear charge. The additional protons’ influence outweighs any minimal additional shielding offered by the electrons in the same shell.
Exceptions and Nuances
While the general trend of decreasing atomic radius across a period is consistent, there are some exceptions and nuances to consider:
-
Transition Metals: The decrease in atomic radius across the transition metal series is less pronounced than in other periods. This is because the added electrons are filling inner d orbitals, which are less effective at shielding the outer electrons than electrons in the outermost s or p orbitals. The increasing nuclear charge still influences the atomic radius, but the effect is less dramatic.
-
Lanthanides and Actinides: The decrease in atomic radius across these series is also less dramatic than in other periods, due to the similar shielding effects and complex orbital filling patterns. The lanthanide contraction, a phenomenon where the atomic radius decreases unexpectedly across the lanthanide series, further complicates this trend.
-
Anomalous Electron Configurations: Some elements exhibit slightly different electron configurations than expected, leading to minor deviations from the general trend. These exceptions are usually subtle and do not significantly alter the overall pattern.
The Importance of Understanding Atomic Radius Trends
Understanding the trend of decreasing atomic radius across a period is crucial for predicting and explaining various chemical properties:
-
Ionization Energy: The energy required to remove an electron from an atom. Atoms with smaller radii generally have higher ionization energies because the outer electrons are held more tightly by the nucleus.
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Atoms with smaller radii tend to be more electronegative, as their outer electrons are more strongly attracted to their nuclei.
-
Reactivity: Atomic radius significantly influences the reactivity of elements. For example, the smaller size of halogens leads to their high reactivity due to their stronger attraction for electrons.
Conclusion: A Comprehensive View
The decrease in atomic radius across a period is a fundamental periodic trend directly linked to the interplay between increasing nuclear charge and the relatively ineffective shielding provided by electrons added to the same energy level. While exceptions exist, understanding the dominant role of effective nuclear charge provides a robust explanation for this key observation in the periodic table. This knowledge underpins our understanding of various other crucial chemical properties and reactivities, making it a cornerstone of chemical prediction and explanation. Further exploration of this topic delves into the nuances and exceptions, providing a more complete picture of atomic structure and periodic trends. The interplay of these forces reveals the intricate beauty and predictability of the chemical world.
Latest Posts
Latest Posts
-
Angular Distance North Or South Of The Equator
Apr 02, 2025
-
Is Rubbing Alcohol Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Is Equivalent Fraction Of 4 5
Apr 02, 2025
-
Organelle Where Cellular Respiration Takes Place
Apr 02, 2025
-
What Number Is 45 Of 60
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Does Radius Decrease Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
