17 Protons 18 Neutrons 17 Electrons
News Leon
Mar 30, 2025 · 6 min read
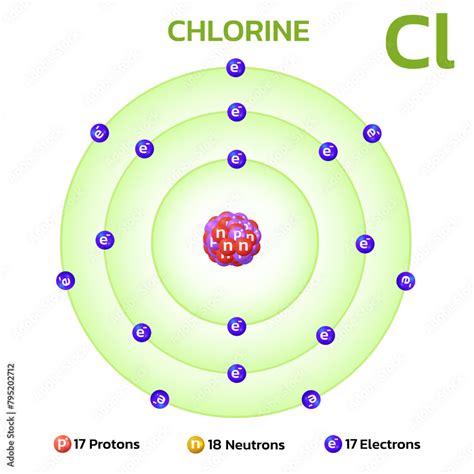
Table of Contents
17 Protons, 18 Neutrons, 17 Electrons: Unveiling the Mystery of Chlorine-35
The seemingly simple combination of 17 protons, 18 neutrons, and 17 electrons paints a fascinating picture in the world of atomic structure and chemistry. This specific arrangement defines Chlorine-35 (³⁵Cl), a stable isotope of chlorine that makes up roughly 76% of all naturally occurring chlorine. Understanding its properties helps us comprehend the behavior of chlorine in various contexts, from its role in everyday life to its crucial applications in diverse industries. This detailed exploration will delve into the intricacies of this isotope, examining its nuclear composition, electronic configuration, chemical properties, and applications.
Delving into the Atomic Structure
The numbers – 17 protons, 18 neutrons, and 17 electrons – provide a blueprint of the atom. Let’s break down each component:
Protons: The Defining Characteristic
The 17 protons are located in the atom's nucleus and define the element as chlorine. The atomic number of an element, which is 17 for chlorine, is equal to the number of protons in its nucleus. This number is fundamental because it dictates the chemical properties of the element. It determines the number of electrons that the atom will possess in its neutral state, and thus, its reactivity.
Neutrons: Adding Stability
The 18 neutrons reside alongside the protons in the nucleus. While they do not affect the element's chemical identity, they significantly contribute to its stability. Neutrons help to bind the positively charged protons together, preventing the nucleus from repelling itself apart. Chlorine-35, with its 18 neutrons, is a stable isotope, meaning its nucleus is unlikely to undergo radioactive decay. Isotopes with different neutron counts (like Chlorine-37 which has 20 neutrons) can exhibit different stability characteristics.
Electrons: The Chemical Players
The 17 electrons orbit the nucleus in specific energy levels or shells. These electrons are responsible for the chemical behavior of chlorine. In a neutral chlorine atom, the electrons are equal in number to the protons, resulting in a net charge of zero. Chlorine's electronic configuration is [Ne] 3s²3p⁵, indicating its tendency to gain one electron to achieve a stable octet configuration, similar to the noble gas argon. This drives its high reactivity and its role as a strong oxidizing agent.
Chemical Properties and Reactivity
The arrangement of electrons dictates chlorine's chemical properties and high reactivity. As mentioned, chlorine has a strong tendency to gain one electron to achieve a stable electron configuration. This tendency makes it highly reactive and a powerful oxidizing agent, meaning it readily accepts electrons from other atoms or molecules.
Oxidation and Reduction
Chlorine's ability to oxidize other substances is a critical aspect of its chemistry. When chlorine gains an electron, it undergoes reduction, and the substance that loses the electron is oxidized. This redox reaction is central to many of chlorine's applications. The reaction between chlorine and sodium, for example, which produces sodium chloride (common table salt), is a classic example of a redox reaction.
Electronegativity and Bonding
Chlorine has a high electronegativity, meaning it strongly attracts electrons in a chemical bond. This results in the formation of polar covalent bonds when it combines with other atoms, particularly those with lower electronegativity. This polarity contributes significantly to the properties of many chlorine-containing compounds.
Formation of Ions and Compounds
Because of its strong tendency to gain an electron, chlorine readily forms the chloride ion (Cl⁻), a negatively charged ion with a stable octet configuration. This ion is found in numerous ionic compounds, including sodium chloride (NaCl), potassium chloride (KCl), and calcium chloride (CaCl₂). The formation of these compounds plays a crucial role in various chemical processes and biological functions.
Isotopes of Chlorine: A Closer Look at ³⁵Cl and ³⁷Cl
Chlorine exists in nature as a mixture of two stable isotopes: Chlorine-35 (³⁵Cl) and Chlorine-37 (³⁷Cl). While both share the same number of protons (17), they differ in their number of neutrons. ³⁵Cl, as discussed extensively, has 18 neutrons, while ³⁷Cl has 20 neutrons.
The difference in neutron number has minimal effect on the chemical behavior of the two isotopes, as chemical properties are primarily determined by the number of protons and electrons. However, the difference in mass does affect the physical properties such as density and diffusion rates. The relative abundance of ³⁵Cl (around 76%) and ³⁷Cl (around 24%) affects the average atomic mass of chlorine, which is approximately 35.45 amu (atomic mass units).
Applications of Chlorine and its Compounds
The unique properties of chlorine and its compounds lead to a wide range of applications across various industries. These range from everyday uses to specialized technological applications.
Water Purification: A Vital Role
One of the most well-known applications of chlorine is in water purification. Chlorine's strong oxidizing power makes it effective in killing harmful bacteria, viruses, and other microorganisms present in water supplies. This ensures the safety and potability of drinking water and prevents the spread of waterborne diseases. This application significantly impacts public health worldwide.
Industrial Applications: Beyond Water Treatment
Chlorine also finds extensive use in various industrial processes:
- Production of PVC (Polyvinyl Chloride): Chlorine is a key component in the manufacturing of polyvinyl chloride (PVC), a widely used plastic material in construction, packaging, and other applications.
- Bleaching Agent: Chlorine's oxidizing power makes it an effective bleaching agent in the paper and textile industries.
- Disinfectants: Chlorine-based compounds are used as disinfectants in hospitals and other settings to sanitize surfaces and equipment, preventing the spread of infections.
- Solvent Production: Chlorine is used in the production of various solvents used in different industries.
Medical and Pharmaceutical Applications
Chlorine and its compounds also have medical and pharmaceutical applications:
- Electrolytes: Chloride ions are essential electrolytes in the human body, playing a vital role in maintaining fluid balance and nerve function.
- Medications: Some medications contain chlorine or chlorine-containing compounds.
Environmental Considerations: A Balanced Perspective
While chlorine has numerous benefits, its use necessitates careful consideration of environmental impacts. Some chlorine-containing compounds can be harmful to the environment and human health if not handled properly. It's crucial to implement appropriate safety measures and responsible disposal methods to minimize any negative effects. Research into more sustainable alternatives and improved handling practices is continuously ongoing.
Conclusion: A Multifaceted Element
The seemingly simple combination of 17 protons, 18 neutrons, and 17 electrons within a chlorine-35 atom underpins its unique characteristics and wide-ranging applications. Its strong oxidizing power, high electronegativity, and the ability to form stable chloride ions make it indispensable in various industries. From purifying drinking water and manufacturing plastics to its role in medicine, chlorine's significance is undeniable. However, responsible use and environmental consciousness are paramount to ensure the sustainable and safe application of this versatile element. Further research into chlorine's behavior and its interactions with other elements continues to expand our understanding and enable the development of innovative applications while mitigating potential environmental risks. The journey of understanding this atom – seemingly simple yet surprisingly complex – continues to evolve.
Latest Posts
Latest Posts
-
Synaptic Knobs Are At The End Of
Apr 01, 2025
-
What Is The Opposite Of Brilliant
Apr 01, 2025
-
Does Accumulated Depreciation Have A Credit Balance
Apr 01, 2025
-
What Element Has 4 Protons And 5 Neutrons
Apr 01, 2025
-
Why Does Radius Decrease Across A Period
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about 17 Protons 18 Neutrons 17 Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
