How Many Electrons Does Antimony Have
News Leon
Mar 30, 2025 · 5 min read
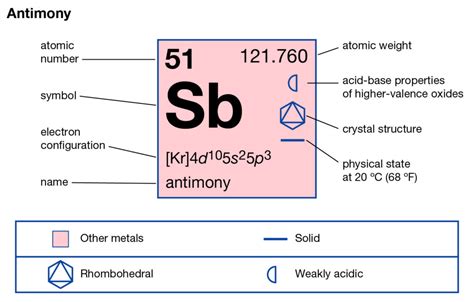
Table of Contents
How Many Electrons Does Antimony Have? A Deep Dive into Atomic Structure
Antimony, a metalloid with a fascinating array of properties and applications, holds a unique place in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is crucial to comprehending its chemical behavior and technological significance. This article will delve deep into the electron configuration of antimony, exploring its position within the periodic table, its valence electrons, and the implications of its electronic structure for its various applications.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before focusing on antimony's electrons, let's briefly review the fundamental components of an atom. Every atom consists of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons is typically equal to the number of protons in a neutral atom. These electrons determine an atom's chemical properties and how it interacts with other atoms.
Antimony's Place in the Periodic Table: Unveiling its Atomic Number
Antimony (Sb), with its atomic number of 51, sits in Group 15 (also known as the pnictogens) and Period 5 of the periodic table. Its atomic number, 51, directly tells us that a neutral antimony atom possesses 51 electrons. This fundamental fact underpins all of its chemical and physical characteristics.
Electron Configuration: Distributing Electrons in Shells and Subshells
The electrons within an atom aren't randomly scattered; they occupy specific energy levels, also known as shells and subshells. These shells are designated by principal quantum numbers (n = 1, 2, 3, etc.), and subshells within each shell are labeled s, p, d, and f. The electron configuration provides a detailed description of how the electrons are distributed among these shells and subshells.
Antimony's electron configuration is: 1s²2s²2p⁶3s²3p⁶3d¹⁰4s²4p⁶4d¹⁰5s²5p³.
Let's break this down:
- 1s²: Two electrons in the first shell's s subshell.
- 2s²2p⁶: Eight electrons in the second shell (two in the s subshell and six in the p subshell).
- 3s²3p⁶3d¹⁰: Eighteen electrons in the third shell.
- 4s²4p⁶4d¹⁰: Eighteen electrons in the fourth shell.
- 5s²5p³: Five electrons in the fifth shell.
This detailed arrangement explains the atom's chemical reactivity and its position in the periodic table.
Understanding the Significance of Valence Electrons
The outermost electrons, those in the highest energy level, are called valence electrons. These electrons are crucial in determining an atom's chemical bonding behavior. In antimony's case, the valence electrons are the five electrons in the 5s and 5p subshells (5s²5p³). This configuration explains antimony's ability to form various compounds with different oxidation states, ranging from -3 to +5.
Antimony's Chemical Behavior: The Role of Electrons
The presence of five valence electrons explains antimony's diverse chemical behavior:
-
Formation of covalent bonds: Antimony readily shares its valence electrons with other atoms to achieve a stable electron configuration, usually resembling a noble gas. This is the basis of its covalent bonding in many compounds. For instance, in stibine (SbH₃), antimony shares three electrons with three hydrogen atoms to complete its octet.
-
Formation of ionic bonds: Under certain conditions, antimony can lose or gain electrons to form ionic bonds. For example, in antimony trioxide (Sb₂O₃), antimony exhibits a +3 oxidation state, losing three electrons.
-
Variable oxidation states: The availability of five valence electrons allows antimony to exhibit variable oxidation states, typically +3 and +5. This variability leads to the formation of a wide range of compounds with diverse properties.
Applications of Antimony: From Alloys to Flame Retardants
The unique electronic structure of antimony contributes directly to its widespread applications across various industries:
-
Alloys: Antimony is added to lead to improve its hardness and mechanical strength, leading to its use in batteries, cable sheathing, and bullets. The interaction of antimony's electrons with those of lead enhances the alloy's overall properties.
-
Flame retardants: Antimony trioxide (Sb₂O₃) is a crucial component in various flame-retardant materials. Its effectiveness stems from its ability to interfere with the combustion process at a molecular level, influenced by its electron configuration and interactions with other molecules during the burning process.
-
Semiconductors: Antimony's semiconducting properties, directly related to its electron configuration and energy band structure, find applications in specialized electronic components.
-
Pharmaceuticals: Certain antimony compounds have historical and ongoing use in medicinal applications, though their use is becoming less frequent due to toxicity concerns. Their interaction within biological systems is dependent on their electronic structure and ability to bind with other molecules.
Isotopes of Antimony: Variations in Neutron Number
While the number of electrons in a neutral antimony atom remains constant at 51, the number of neutrons can vary. This leads to the existence of different isotopes of antimony, such as ¹²¹Sb and ¹²³Sb, which differ in their mass numbers (total number of protons and neutrons). Despite the variation in neutron number, the number of electrons in a neutral atom of any antimony isotope remains 51.
Conclusion: The Significance of Antimony's 51 Electrons
The seemingly simple statement that antimony has 51 electrons actually encapsulates the essence of this element's behavior and applications. The detailed electron configuration dictates its chemical reactivity, its capacity to form diverse compounds, and ultimately, its usefulness in various technologies. Understanding the number and arrangement of these electrons is paramount to understanding the intriguing properties and versatile applications of this remarkable metalloid. Further research into the intricacies of antimony's electronic structure continues to unveil new possibilities and applications in various scientific fields. From its role in alloys to its use in flame retardants, the 51 electrons of antimony are central to its significant contribution to modern society.
Latest Posts
Latest Posts
-
Why Does Radius Decrease Across A Period
Apr 01, 2025
-
The Krebs Cycle Takes Place In
Apr 01, 2025
-
What Is The Following Sum In Simplest Form
Apr 01, 2025
-
Weak Acid And Weak Base Ph
Apr 01, 2025
-
What Is The Measure Of Angle B In Degrees
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Antimony Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
