Why Is Fluorine More Electronegative Than Chlorine
News Leon
Apr 01, 2025 · 5 min read
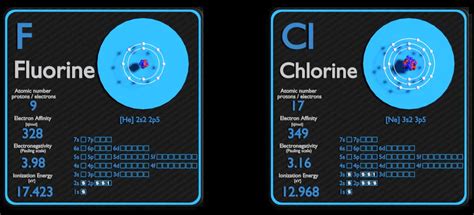
Table of Contents
Why is Fluorine More Electronegative Than Chlorine? A Deep Dive into Electronegativity
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons within a chemical bond. While it might seem intuitive that larger atoms like chlorine would be more electronegative due to their greater number of electrons, the reality is quite different. Fluorine, significantly smaller than chlorine, boasts the highest electronegativity of all elements. This seemingly paradoxical behavior stems from a complex interplay of several factors, which we will explore in detail.
Understanding Electronegativity: A Foundation
Before delving into the comparison between fluorine and chlorine, let's establish a solid understanding of electronegativity. It's not a directly measurable quantity but rather a relative property, often represented using the Pauling scale, where fluorine is assigned a value of 4.0. Other elements are then ranked relative to fluorine.
Several factors contribute to an atom's electronegativity:
-
Effective Nuclear Charge: This refers to the net positive charge experienced by valence electrons. A higher effective nuclear charge means the nucleus exerts a stronger pull on the electrons. This is crucial in understanding the fluorine-chlorine difference.
-
Atomic Radius: The distance between the nucleus and the valence electrons. A smaller atomic radius leads to a stronger attraction between the nucleus and the valence electrons, increasing electronegativity.
-
Shielding Effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus. The more inner electrons, the weaker the effective nuclear charge felt by the valence electrons.
Fluorine's Reign: Unpacking the Superior Electronegativity
Fluorine's exceptional electronegativity arises from a potent combination of these factors:
1. Small Atomic Radius: A Powerful Proximity
Fluorine possesses the smallest atomic radius among all halogens. This means its valence electrons are significantly closer to the positively charged nucleus. The shorter the distance, the stronger the electrostatic attraction, leading to a much greater pull on shared electrons in a chemical bond. This proximity effect significantly outweighs the influence of increased electron-electron repulsion in smaller atoms.
2. High Effective Nuclear Charge: A Strong Pull
Despite having only nine protons, fluorine exhibits a surprisingly high effective nuclear charge. While it has fewer protons than chlorine (17), the smaller size of the fluorine atom minimizes shielding effects. The inner electrons are less effective at shielding the valence electrons from the positive charge of the nucleus. This results in a stronger net positive charge experienced by the outermost electrons, enhancing the attraction to shared electrons in a bond.
3. Minimal Shielding: Direct Nuclear Influence
The compact electron configuration of fluorine minimizes the shielding effect. The relatively few inner electrons do not effectively shield the valence electron from the nucleus's pull. In contrast, chlorine, with its larger number of inner electrons, experiences a greater shielding effect. This weakens the effective nuclear charge experienced by chlorine's valence electrons, reducing its electronegativity compared to fluorine.
Chlorine's Position: Why it Falls Short
While chlorine is also highly electronegative, it lacks the compelling combination of factors that propel fluorine to the top. Let's examine why:
1. Larger Atomic Radius: Diminished Attraction
Chlorine possesses a significantly larger atomic radius than fluorine. This increased distance between the nucleus and valence electrons weakens the electrostatic attraction. The greater separation diminishes the nucleus's ability to effectively pull on shared electrons during bonding, resulting in lower electronegativity.
2. Increased Shielding: Weakened Nuclear Pull
Chlorine's greater number of inner electrons leads to a more substantial shielding effect. These inner electrons effectively block some of the positive charge of the nucleus from reaching the valence electrons. This reduces the effective nuclear charge experienced by the valence electrons, subsequently decreasing the atom's ability to attract shared electrons in a bond.
3. Increased Electron-Electron Repulsion: A Competing Force
With more electrons in its electron shells, chlorine experiences stronger electron-electron repulsion. This repulsion counteracts the attractive force exerted by the nucleus on the valence electrons. In fluorine, the smaller size mitigates this repulsion, allowing the nuclear attraction to dominate.
The Comparative Analysis: A Clear Picture
To summarize the comparison, let's outline the key differences in a table:
| Feature | Fluorine | Chlorine |
|---|---|---|
| Atomic Radius | Very small | Larger |
| Effective Nuclear Charge | High | Lower |
| Shielding Effect | Minimal | Significant |
| Electron-Electron Repulsion | Low | Higher |
| Electronegativity | Highest (4.0 on Pauling scale) | High (3.0 on Pauling scale) |
Beyond the Basics: Implications and Applications
The difference in electronegativity between fluorine and chlorine has significant implications across various aspects of chemistry:
-
Bond Polarity: Fluorine's high electronegativity results in highly polar bonds when it forms covalent bonds with other elements. This polarity has significant implications for the reactivity and properties of the resulting compounds.
-
Bond Strength: The strong attraction of fluorine to electrons contributes to the formation of relatively strong chemical bonds.
-
Chemical Reactivity: Fluorine's exceptional electronegativity makes it one of the most reactive elements in the periodic table. Its high reactivity stems from its powerful tendency to gain an electron and achieve a stable octet configuration.
-
Industrial Applications: Fluorine's unique properties are exploited in various industrial processes, including the production of fluorocarbons (used in refrigerants and other applications), and in the synthesis of numerous pharmaceuticals and other specialty chemicals.
-
Biological Significance: Fluorine's role in biological systems is less prominent compared to other halogens, but its presence in certain compounds and its effects on biological processes are areas of ongoing research.
Conclusion: A Triumph of Size and Charge
The superior electronegativity of fluorine compared to chlorine is not a simple matter of atomic number. Instead, it is a compelling illustration of the complex interplay between atomic radius, effective nuclear charge, and shielding effects. Fluorine's remarkably small size and its high effective nuclear charge, combined with minimal shielding, result in an exceptionally strong pull on electrons, making it the undisputed champion of electronegativity among the elements. Understanding this fundamental principle is crucial for grasping the reactivity, bonding, and diverse applications of both fluorine and chlorine in chemistry and related fields.
Latest Posts
Latest Posts
-
Does Reactivity Increase Down A Group
Apr 02, 2025
-
How Many Vertices Does Octagon Have
Apr 02, 2025
-
Reaction Of Ammonia And Sulfuric Acid
Apr 02, 2025
-
What Type Of Triangle Is Shown Below
Apr 02, 2025
-
Which Of The Following Pairs Are Mismatched
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Is Fluorine More Electronegative Than Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
