Who Developed Planetary Model Of The Atom
News Leon
Mar 28, 2025 · 6 min read
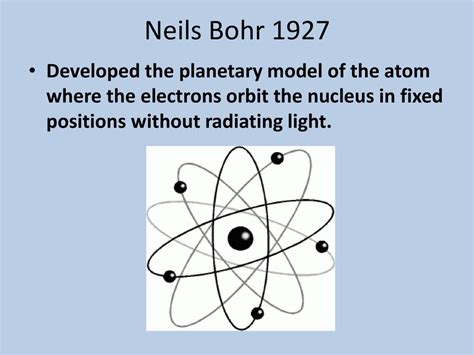
Table of Contents
Who Developed the Planetary Model of the Atom? A Journey Through Atomic Theory
The image of the atom as a miniature solar system, with electrons orbiting a central nucleus, is deeply ingrained in our collective understanding. This iconic "planetary model" of the atom is arguably one of the most recognizable scientific images, instantly conjuring visions of whirling electrons and a dense, positively charged core. But who deserves the credit for this revolutionary conceptualization? The answer isn't straightforward, as the development of the planetary model was a collaborative, iterative process spanning several decades and involving multiple brilliant minds. This article will delve into the key figures and their contributions, examining the evolution of atomic theory leading to the model we know today.
From Dalton's Solid Spheres to Thomson's Plum Pudding
Before we can understand the planetary model, we need to appreciate the foundations upon which it was built. John Dalton's atomic theory, proposed in the early 1800s, posited that all matter is composed of indivisible and indestructible atoms, each element having its own unique atomic weight. This marked a significant leap forward but lacked any internal structure for the atom. Atoms were simply considered solid, indivisible spheres.
This view was dramatically altered by J.J. Thomson's discovery of the electron in 1897. Thomson's experiments with cathode rays demonstrated the existence of negatively charged particles far smaller than the atom itself. This discovery shattered Dalton's notion of the indivisible atom, forcing scientists to rethink the atom's structure. Thomson proposed the "plum pudding" model, a sphere of positive charge with negatively charged electrons embedded within it, like plums in a pudding. This was a significant step, acknowledging the existence of subatomic particles, but it still lacked the crucial spatial organization that would define the planetary model.
Rutherford's Gold Foil Experiment: A Revolutionary Breakthrough
The true architect of the planetary model is widely considered to be Ernest Rutherford. In 1909, Rutherford, along with his students Hans Geiger and Ernest Marsden, conducted a groundbreaking experiment that would forever change our understanding of the atom. The experiment involved bombarding a thin gold foil with alpha particles (positively charged particles). While most alpha particles passed straight through the foil, a small but significant number were deflected at large angles, some even bouncing directly back.
This unexpected result could not be explained by Thomson's plum pudding model. If the positive charge were uniformly distributed throughout the atom, as Thomson proposed, the alpha particles would not experience such significant deflections. Rutherford's brilliant interpretation of these results led to the proposal of a new atomic model: the atom consists of a tiny, dense, positively charged nucleus at its center, containing most of the atom's mass, with the electrons orbiting this nucleus at a considerable distance.
Rutherford's model successfully explained the experimental observations:
- Most alpha particles passed straight through: The vast majority of the atom's volume is empty space.
- Some alpha particles were deflected at large angles: These particles encountered the dense, positively charged nucleus, causing repulsion and deflection.
- A few alpha particles bounced directly back: These particles collided head-on with the nucleus, experiencing a strong repulsive force.
Rutherford's announcement of this new model in 1911 was a paradigm shift in atomic physics. It replaced the static and somewhat simplistic plum pudding model with a dynamic model that captured the atom's internal structure with unprecedented clarity. The implications were enormous, revolutionizing our understanding of matter at the fundamental level.
The Limitations of Rutherford's Planetary Model and the Path to Quantum Mechanics
While Rutherford's model was a monumental advancement, it had significant limitations. Classical electromagnetism predicted that accelerating charged particles, like electrons orbiting the nucleus, would emit electromagnetic radiation, losing energy and spiraling into the nucleus. This would lead to the atom's collapse, a phenomenon that clearly doesn't occur in reality. This inconsistency highlighted the inadequacy of classical physics in describing the atom's behavior.
The resolution to this problem would come with the advent of quantum mechanics. Niels Bohr, building upon Rutherford's model, introduced his own atomic model in 1913. Bohr's model incorporated the revolutionary concept of quantized energy levels, proposing that electrons could only occupy specific orbits at fixed distances from the nucleus. Electrons could jump between these orbits by absorbing or emitting photons of specific energies, explaining the discrete nature of atomic spectra. While still a planetary model in its fundamental structure, Bohr's model addressed the instability problem by incorporating quantum principles.
Beyond Bohr: The Refinement of the Planetary Model
Bohr's model, while a significant improvement, was still incomplete. It could not accurately predict the spectra of atoms with more than one electron. The development of quantum mechanics in the 1920s, spearheaded by figures like Werner Heisenberg, Erwin Schrödinger, and Max Born, provided a more sophisticated and accurate framework for understanding atomic structure and behavior. The Schrödinger equation, a fundamental equation in quantum mechanics, allows for the calculation of electron wave functions, describing the probability of finding an electron at a particular location around the nucleus.
This led to the modern quantum mechanical model of the atom, where electrons are described not as orbiting particles in specific orbits, but rather as existing in probability clouds or orbitals, representing regions of space where the probability of finding an electron is high. This model is significantly more complex than the simple planetary model but retains the core concept of a central nucleus surrounded by electrons. The planetary model, while conceptually useful and visually appealing, is essentially a simplified representation of the far more intricate reality described by quantum mechanics.
The Legacy of the Planetary Model
Despite its limitations in accurately reflecting the true behavior of electrons, the planetary model remains an invaluable tool for understanding basic atomic structure. Its simplicity and intuitive visual representation make it a powerful pedagogical tool, allowing students to grasp the fundamental concepts of a central nucleus and orbiting electrons. It serves as a stepping stone to the more sophisticated quantum mechanical model, providing a foundational understanding before delving into the complexities of wave functions and probability distributions.
Furthermore, the planetary model’s historical significance cannot be overstated. It marks a pivotal point in the history of science, representing a dramatic shift in our understanding of matter. The journey from Dalton's indivisible spheres to Rutherford's nucleus and orbiting electrons, and finally to the nuanced world of quantum mechanics, illustrates the dynamic and iterative nature of scientific progress.
Conclusion: A Collaborative Effort
In conclusion, attributing the development of the planetary model to a single individual is an oversimplification. The model emerged as a culmination of the work of several prominent scientists, each building upon the contributions of their predecessors. John Dalton laid the groundwork with his atomic theory, J.J. Thomson discovered the electron, and Ernest Rutherford's gold foil experiment provided the crucial experimental evidence that led to the conceptualization of the nucleus and orbiting electrons. Niels Bohr then incorporated quantum principles to address the limitations of Rutherford's model. While the quantum mechanical model ultimately superseded it, Rutherford's model remains a milestone in the history of atomic theory, a testament to scientific inquiry and a powerful tool for understanding the fundamental building blocks of the universe. The legacy of the planetary model lies not only in its accuracy but also in its role as a crucial stepping stone in our journey towards understanding the quantum world. Its enduring image serves as a lasting symbol of the ongoing pursuit of knowledge in the fascinating realm of atomic physics.
Latest Posts
Latest Posts
-
Which Statement About Equations And Expressions Is True
Mar 31, 2025
-
Points On The Same Line Are Called
Mar 31, 2025
-
Are Tsunami Waves Transverse Or Longitudinal
Mar 31, 2025
-
How To Balance A Nuclear Equation
Mar 31, 2025
-
What Happens To Voltage If Resistance Increases
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Who Developed Planetary Model Of The Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
