Which Of The Following Is An Endothermic Process
News Leon
Mar 31, 2025 · 5 min read
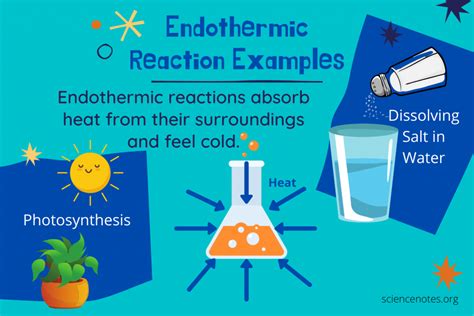
Table of Contents
Which of the Following is an Endothermic Process? Understanding Energy Changes in Chemical Reactions
Understanding endothermic and exothermic processes is fundamental to grasping the principles of thermodynamics and chemistry. While many chemical reactions release heat (exothermic), others require an input of heat to proceed (endothermic). This article delves into the definition of endothermic processes, explores various examples, and provides a clear understanding of how to identify them. We'll also tackle common misconceptions and offer practical applications to solidify your understanding.
Defining Endothermic Processes: Absorbing Energy from the Surroundings
An endothermic process is any process that absorbs heat from its surroundings. Think of it like a sponge soaking up water – the process (the sponge absorbing water) requires energy input (the water). Similarly, an endothermic reaction absorbs energy in the form of heat, causing a decrease in the temperature of the surroundings. This energy is used to break chemical bonds in the reactants, leading to the formation of products with higher potential energy.
Key Characteristics of Endothermic Processes:
- Heat absorption: The most defining feature is the absorption of heat energy. This leads to a cooling effect on the surroundings.
- Positive enthalpy change (ΔH > 0): Enthalpy (H) represents the total heat content of a system. A positive change indicates that the system has gained heat.
- Higher potential energy of products: The products of an endothermic reaction possess higher potential energy compared to the reactants.
- Often non-spontaneous: Many endothermic reactions are non-spontaneous, meaning they require an external energy input to proceed.
Examples of Endothermic Processes: From Everyday Life to Industrial Applications
Endothermic processes aren't just confined to the laboratory; they occur frequently in everyday life and have significant industrial applications. Let's explore several compelling examples:
1. Photosynthesis: The Engine of Life
Photosynthesis, the process by which plants convert light energy into chemical energy, is a classic example of an endothermic process. Plants absorb sunlight (energy) to convert carbon dioxide and water into glucose (a sugar) and oxygen. This process requires a significant input of energy to break the strong bonds in carbon dioxide and water and form the new bonds in glucose. The temperature of the surrounding environment decreases slightly as the plant absorbs energy for photosynthesis.
2. Melting Ice: A Phase Transition
Melting ice cubes is another readily observable endothermic process. To change from a solid (ice) to a liquid (water), the ice must absorb heat from its surroundings. This heat energy is used to overcome the intermolecular forces holding the water molecules together in the rigid ice structure. The surrounding air cools as the ice melts, absorbing heat from the air.
3. Evaporating Water: Cooling Effect
The evaporation of water is an endothermic process. When water molecules transition from the liquid phase to the gaseous phase, they absorb energy to overcome the attractive forces between them. This is why sweating cools us down – the evaporation of sweat absorbs heat from our skin, lowering our body temperature.
4. Cooking an Egg: Denaturation of Proteins
Cooking an egg involves denaturation of proteins, which is an endothermic process. Heat is absorbed to break the weak bonds maintaining the protein's three-dimensional structure. The egg white changes from a clear liquid to a solid white as the heat is absorbed.
5. Dissolving Ammonium Nitrate in Water: A Common Demonstration
Dissolving ammonium nitrate (NH₄NO₃) in water is a widely used demonstration of an endothermic reaction in chemistry classrooms. When ammonium nitrate dissolves, it absorbs heat from the surrounding water, resulting in a noticeable decrease in the water's temperature. The container often feels cold to the touch.
Distinguishing Endothermic from Exothermic Processes: Key Differences
It's crucial to differentiate endothermic processes from their counterparts, exothermic processes. Exothermic processes release heat to the surroundings, resulting in an increase in temperature. Here’s a table summarizing the key differences:
| Feature | Endothermic Process | Exothermic Process |
|---|---|---|
| Heat Transfer | Absorbs heat from surroundings | Releases heat to surroundings |
| Enthalpy Change (ΔH) | Positive (ΔH > 0) | Negative (ΔH < 0) |
| Temperature Change | Decrease in surrounding temperature | Increase in surrounding temperature |
| Energy of Products | Products have higher potential energy than reactants | Products have lower potential energy than reactants |
| Examples | Photosynthesis, melting ice, evaporating water | Combustion, neutralization reactions, rust formation |
Common Misconceptions about Endothermic Processes
Several misconceptions often surround endothermic processes. Let's address them:
- Endothermic reactions are always slow: The speed of a reaction is determined by its activation energy, not whether it's endothermic or exothermic. Some endothermic reactions can be fast, while others are slow.
- Endothermic reactions are always inefficient: While they require energy input, endothermic processes are essential for many crucial life processes and industrial applications. Their usefulness isn't determined solely by energy input.
- Feeling cold always indicates an endothermic process: While many endothermic processes cause a cooling effect, some exothermic processes can also feel cold initially due to factors like rapid evaporation or expansion.
Practical Applications of Understanding Endothermic Processes
The understanding of endothermic processes has numerous practical applications across various fields:
- Chemistry: Designing chemical reactions, controlling reaction rates, and developing new materials.
- Biology: Understanding metabolic processes, such as photosynthesis and cellular respiration.
- Engineering: Developing efficient cooling systems, designing energy-efficient buildings, and improving industrial processes.
- Medicine: Understanding drug delivery systems and the effects of certain medical treatments.
Conclusion: Embracing the Endothermic World
Endothermic processes, while requiring energy input, are crucial to various natural and industrial processes. From the life-sustaining process of photosynthesis to the simple act of melting ice, these processes are fundamental to our understanding of energy transfer and chemical transformations. By understanding their key characteristics and differentiating them from exothermic processes, we can appreciate their importance and apply this knowledge across various scientific and technological fields. This understanding allows us to innovate and develop new solutions in areas like renewable energy, material science, and medicine, shaping a more sustainable and technologically advanced future.
Latest Posts
Latest Posts
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
-
Lewis Dot Structure For Magnesium Chloride
Apr 01, 2025
-
A Group Of Related Records Is Called A Table
Apr 01, 2025
-
Provides Long Term Energy Storage For Animals
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Endothermic Process . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
