Lewis Dot Structure For Magnesium Chloride
News Leon
Apr 01, 2025 · 5 min read
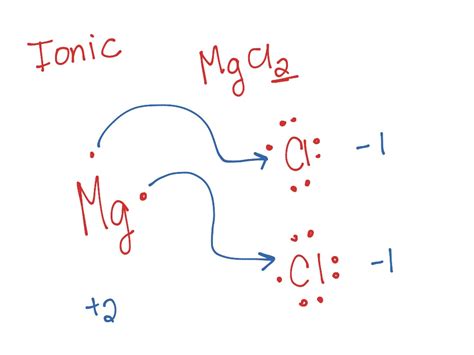
Table of Contents
Lewis Dot Structure for Magnesium Chloride: A Comprehensive Guide
Magnesium chloride (MgCl₂), a common ionic compound, provides an excellent example for understanding Lewis dot structures, a fundamental concept in chemistry. This article will delve deep into constructing the Lewis dot structure for MgCl₂, explaining the underlying principles, step-by-step procedures, and the implications of its structure on the compound's properties. We'll also explore related concepts to build a comprehensive understanding.
Understanding Lewis Dot Structures
Before diving into MgCl₂, let's clarify what Lewis dot structures represent. A Lewis dot structure, also known as an electron dot diagram, is a visual representation of the valence electrons of atoms within a molecule. Valence electrons are the electrons located in the outermost shell of an atom and are crucial in chemical bonding. These structures help us visualize how atoms share or transfer electrons to achieve a stable electron configuration, usually a full octet (eight electrons) for main group elements.
Determining Valence Electrons for Magnesium and Chlorine
The first step in constructing any Lewis dot structure is identifying the number of valence electrons for each atom involved. This information is readily available from the periodic table's group number (for main group elements).
-
Magnesium (Mg): Magnesium belongs to Group 2 (alkaline earth metals), meaning it has two valence electrons.
-
Chlorine (Cl): Chlorine is in Group 17 (halogens) and possesses seven valence electrons.
Constructing the Lewis Dot Structure for MgCl₂: A Step-by-Step Guide
-
Identify the central atom: In ionic compounds like MgCl₂, the less electronegative element typically acts as the central atom. Since magnesium is less electronegative than chlorine, it's considered the central atom (though in this case, a central atom isn't really a central concept since it’s an ionic compound rather than a covalent compound).
-
Represent valence electrons: Represent the valence electrons of each atom using dots around their respective chemical symbols. Magnesium (Mg) will have two dots, and each chlorine (Cl) atom will have seven dots.
Mg: •• Cl: •••••••• Cl: •••••••• -
Electron transfer: Magnesium, aiming for a stable octet (though it achieves a stable duet in this case), needs to lose its two valence electrons. Each chlorine atom, to achieve a stable octet, needs to gain one electron. Therefore, magnesium will transfer one electron to each of the two chlorine atoms.
-
Formation of ions: After the electron transfer, magnesium loses two electrons, forming a positively charged magnesium ion (Mg²⁺), and each chlorine atom gains one electron, forming negatively charged chloride ions (Cl⁻).
Mg²⁺ Cl⁻ Cl⁻ -
Representing the ionic bond: The electrostatic attraction between the positively charged Mg²⁺ ion and the negatively charged Cl⁻ ions constitutes the ionic bond in MgCl₂. This is typically represented by showing the ions with their charges enclosed in brackets:
[Mg²⁺] [Cl⁻] [Cl⁻]Note that there aren't any dots representing shared electrons because it's an ionic bond. The electrons have been completely transferred, not shared.
Understanding the Ionic Bond in MgCl₂
The Lewis dot structure clearly depicts the ionic bonding in magnesium chloride. The significant electronegativity difference between magnesium (low) and chlorine (high) results in the complete transfer of electrons. This electron transfer leads to the formation of ions with stable electron configurations, obeying the octet rule (or duet rule for magnesium). The strong electrostatic attraction between these oppositely charged ions is what holds the compound together.
Properties of Magnesium Chloride Related to its Lewis Dot Structure
The Lewis dot structure helps us understand the properties of MgCl₂:
-
High melting and boiling points: The strong electrostatic forces between the Mg²⁺ and Cl⁻ ions require considerable energy to overcome, resulting in high melting and boiling points.
-
Crystalline structure: The arrangement of ions in a regular, repeating pattern leads to the formation of a crystalline solid structure.
-
Solubility in water: The polar water molecules can effectively surround and separate the Mg²⁺ and Cl⁻ ions, leading to the solubility of MgCl₂ in water.
-
Conductivity: Molten magnesium chloride or an aqueous solution of magnesium chloride conducts electricity due to the presence of freely moving ions.
Comparing Ionic and Covalent Bonding
It's important to differentiate between ionic and covalent bonding, which are the two primary types of chemical bonds. While MgCl₂ exemplifies ionic bonding, many other compounds exhibit covalent bonding, where electrons are shared between atoms. The Lewis dot structures for covalent compounds would look quite different, showing shared electron pairs represented as lines or pairs of dots between the atoms.
Advanced Concepts: Lattice Energy and Electronegativity
The stability of MgCl₂ is further explained by concepts like lattice energy and electronegativity.
-
Lattice Energy: Lattice energy refers to the energy released when ions come together to form a crystal lattice. The higher the lattice energy, the stronger the ionic bond. In MgCl₂, the high charge density of the Mg²⁺ ion and the relatively large size of the Cl⁻ ion contribute to a significant lattice energy.
-
Electronegativity: Electronegativity is the measure of an atom's ability to attract electrons towards itself in a chemical bond. The large difference in electronegativity between magnesium and chlorine is the driving force behind the complete electron transfer in the formation of MgCl₂.
Applications of Magnesium Chloride
Magnesium chloride finds diverse applications across various industries:
-
De-icing agent: Its ability to lower the freezing point of water makes it a common de-icing agent for roads and sidewalks in winter.
-
Magnesium production: It serves as a precursor in the production of metallic magnesium.
-
Food additive: It's used as a nutritional supplement (magnesium source) and as a firming agent in food processing.
-
Medicine: It has some medical applications, including as a laxative and in intravenous solutions.
-
Fire retardant: Certain magnesium chloride compounds are also used as fire retardants in various materials.
Conclusion: The Significance of the Lewis Dot Structure for MgCl₂
The Lewis dot structure for magnesium chloride is a simple yet powerful tool that provides insights into the compound's bonding, properties, and applications. It beautifully illustrates the principles of ionic bonding and the role of valence electrons in achieving stable electron configurations. Understanding the Lewis dot structure lays a crucial foundation for comprehending more complex chemical concepts and the behaviour of various chemical compounds. Furthermore, the application of MgCl₂ across various sectors highlights its importance in everyday life and various industries. This detailed explanation should provide a clear and comprehensive understanding of this important chemical compound.
Latest Posts
Latest Posts
-
What Is The Area Of The Shaded Figure
Apr 02, 2025
-
Calculate The Molecular Mass Of Koh
Apr 02, 2025
-
A Long And Branched Chain Of Glucose Molecules Is
Apr 02, 2025
-
What Phase Does The Cytoplasm Divide
Apr 02, 2025
-
A Clique Is A Group Of
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Lewis Dot Structure For Magnesium Chloride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
