Which Of The Following Compounds Is Insoluble
News Leon
Apr 01, 2025 · 5 min read
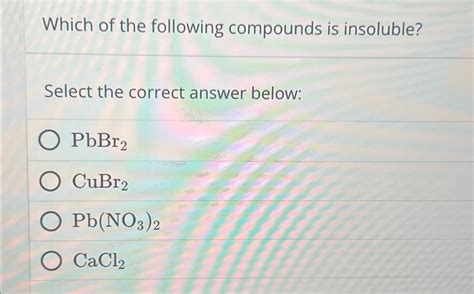
Table of Contents
Which of the Following Compounds is Insoluble? A Comprehensive Guide to Solubility Rules
Solubility, the ability of a substance to dissolve in a solvent, is a fundamental concept in chemistry with far-reaching implications in various fields, from medicine and environmental science to industrial processes and everyday life. Understanding solubility rules is crucial for predicting the outcome of chemical reactions and for designing effective separation techniques. This article delves into the intricacies of solubility, focusing on how to determine which compounds are insoluble. We'll explore the common solubility rules, delve into exceptions, and provide examples to solidify your understanding.
Understanding Solubility and its Factors
Solubility describes the maximum amount of solute that can dissolve in a given amount of solvent at a specific temperature and pressure. A substance is considered soluble if it dissolves readily, insoluble if it dissolves poorly or not at all, and slightly soluble if it dissolves to a limited extent. Several factors influence solubility:
1. Nature of Solute and Solvent:
The "like dissolves like" principle is a cornerstone of solubility. Polar solvents (like water) tend to dissolve polar solutes (ionic compounds and polar molecules), while nonpolar solvents (like hexane) dissolve nonpolar solutes (nonpolar molecules). This is due to the interaction between the intermolecular forces of the solute and solvent. Stronger interactions lead to greater solubility.
2. Temperature:
For most solid solutes, solubility increases with increasing temperature. The added energy increases the kinetic energy of the molecules, facilitating the breaking of solute-solute interactions and allowing greater interaction with the solvent. For gaseous solutes, the opposite is often true; solubility decreases with increasing temperature.
3. Pressure:
Pressure significantly affects the solubility of gases. Higher pressure increases the solubility of gases in liquids, as described by Henry's Law. The effect of pressure on the solubility of solids and liquids is negligible.
4. Common Ion Effect:
The presence of a common ion in a solution decreases the solubility of a sparingly soluble salt. This is because the equilibrium shifts to favor the formation of the undissolved solid.
Common Solubility Rules: Predicting Insoluble Compounds
While there are exceptions, these general solubility rules provide a valuable framework for predicting the solubility of ionic compounds in water:
Rule 1: Most nitrates (NO₃⁻) are soluble.
This is a very reliable rule. Almost all nitrate salts dissolve readily in water. Silver nitrate (AgNO₃) is an exception sometimes used in qualitative analysis.
Rule 2: Most alkali metal (Group 1) salts and ammonium (NH₄⁺) salts are soluble.
Compounds containing lithium (Li⁺), sodium (Na⁺), potassium (K⁺), rubidium (Rb⁺), cesium (Cs⁺), and ammonium (NH₄⁺) ions are generally soluble. There are few exceptions to this broad rule.
Rule 3: Most chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻) salts are soluble.
However, there are important exceptions: salts containing silver (Ag⁺), lead (Pb²⁺), and mercury(I) (Hg₂²⁺) cations are insoluble. Lead chloride (PbCl₂) is slightly soluble in hot water.
Rule 4: Most sulfates (SO₄²⁻) are soluble.
Exceptions include lead(II) sulfate (PbSO₄), barium sulfate (BaSO₄), strontium sulfate (SrSO₄), calcium sulfate (CaSO₄, slightly soluble), and mercury(I) sulfate (Hg₂SO₄). These are often used in gravimetric analysis due to their low solubility.
Rule 5: Most hydroxides (OH⁻) are insoluble.
Exceptions include alkali metal hydroxides and barium hydroxide (Ba(OH)₂). Many transition metal hydroxides are also sparingly soluble.
Rule 6: Most carbonates (CO₃²⁻), phosphates (PO₄³⁻), sulfides (S²⁻), chromates (CrO₄²⁻), and sulfites (SO₃²⁻) are insoluble.
Exceptions are those containing alkali metal cations and ammonium. These anions often form precipitates with many metal cations.
Rule 7: Most fluorides are soluble. But fluorides of the alkaline earth metals and some transition metals are insoluble.
Identifying Insoluble Compounds: Examples and Practice
Let's apply these rules to identify insoluble compounds:
Example 1: Is lead(II) iodide (PbI₂) soluble?
According to Rule 3, most iodides are soluble, but lead(II) is an exception. Therefore, lead(II) iodide is insoluble.
Example 2: Is potassium carbonate (K₂CO₃) soluble?
Rule 6 states that most carbonates are insoluble. However, Rule 2 indicates that most potassium salts are soluble. In this case, Rule 2 takes precedence, making potassium carbonate soluble.
Example 3: Is silver sulfate (Ag₂SO₄) soluble?
Rule 4 indicates that most sulfates are soluble. However, silver is an exception (similar to the chloride rule). Therefore, silver sulfate is insoluble.
Example 4: Is calcium hydroxide Ca(OH)₂ soluble?
Rule 5 dictates that most hydroxides are insoluble. While calcium is an alkaline earth metal, the solubility of calcium hydroxide is low, making it considered insoluble or slightly soluble, depending on the context and the required degree of accuracy.
Example 5: Is ammonium phosphate (NH₄)₃PO₄ soluble?
Rule 6 indicates most phosphates are insoluble. However, Rule 2 shows ammonium salts are generally soluble. Thus, ammonium phosphate is soluble.
Exceptions and Limitations
It's crucial to remember that these rules have exceptions. Solubility is a complex phenomenon influenced by various factors, and these rules are generalizations. For precise predictions, consult solubility product constants (Ksp) values. These constants quantify the solubility of sparingly soluble salts, providing a more accurate measure than simple solubility rules.
Applications of Solubility: Beyond the Lab
The principles of solubility are fundamental to numerous applications:
-
Environmental Science: Understanding solubility helps predict the fate of pollutants in the environment, whether they will remain dissolved or precipitate out of solution.
-
Medicine: Solubility is crucial for drug delivery. Drugs must be soluble enough to be absorbed into the bloodstream but not so soluble that they are rapidly excreted.
-
Industrial Processes: Many industrial processes rely on selective precipitation or dissolution of substances, such as in water purification or metal extraction.
-
Agriculture: Soil solubility determines the availability of nutrients to plants.
Conclusion: Mastering Solubility
Determining which compounds are insoluble requires a solid understanding of solubility rules and their exceptions. While the rules provide a useful framework, it's vital to remember their limitations. Consulting more precise data, such as Ksp values, is necessary for accurate predictions in specific scenarios. By mastering these concepts, you gain a deeper understanding of chemical behavior and its implications across various disciplines. The examples provided throughout this article should help you develop a strong foundation in assessing the solubility of different compounds, a crucial skill in chemistry and related fields. Remember to always consider the specific context and potential exceptions when analyzing the solubility of compounds.
Latest Posts
Latest Posts
-
Why Is The Vacuole Larger In Plant Cells
Apr 02, 2025
-
How To Initialize A Tuple In Python
Apr 02, 2025
-
Find The Acceleration When The Velocity Is 0
Apr 02, 2025
-
Are Metals Solid At Room Temperature
Apr 02, 2025
-
Which Of The Following Statements Correctly Describes Gene Linkage
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Compounds Is Insoluble . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
